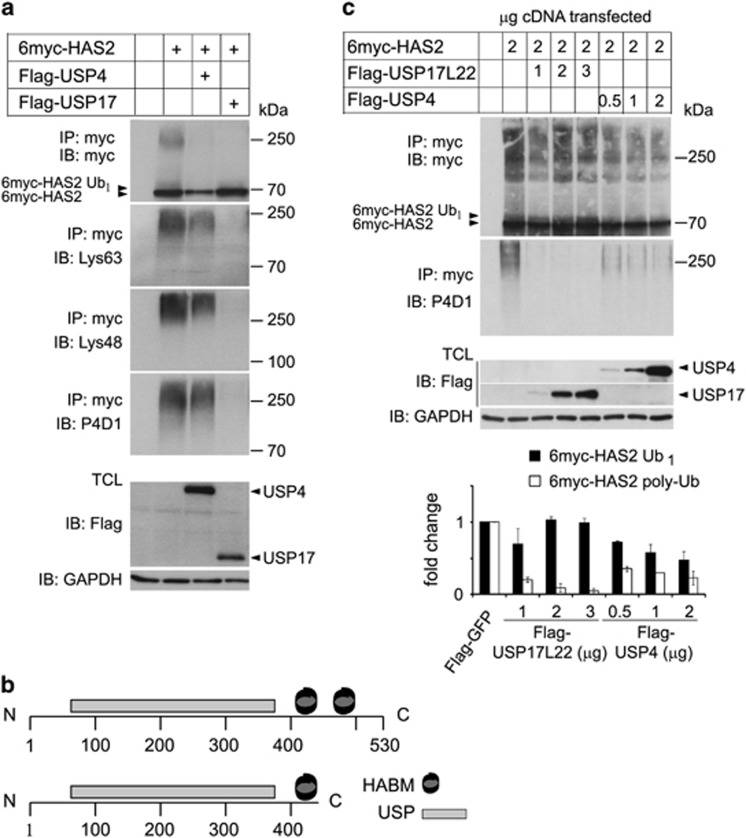
Figure 4.
Lys48 and Lys63 polyubiquitin chains on HAS2 are efficiently removed by USP17, whereas USP4 also removes monoubiquitination. (a) HEK293T cells were co-transfected with 6myc-tagged HAS2 and Flag-tagged USP4 or USP17 cDNAs. 6myc-tagged empty vector and Flag-tagged vector encoding GFP were used as control and to equalize the DNA load. HAS2 was immunoprecipitated after denaturation and immunoblotting was performed with Lys63- or Lys48-specific polyubiquitin antibodies, as well as with P4D1 antibodies. Whole-cell lysates were probed with Flag-M2 antibody to verify DUB expression, and GAPDH was used as loading control. The data shown are a representative experiment out of three performed with similar results. (b) A schematic diagram of the USP17L22 and USP17 isoforms; the ubiquitin-specific protease domain (USP) and the two HABMs at positions 401–409 and 445–453, respectively, are depicted. (c) HEK293T cells were co-transfected with increasing amounts of Flag-USP17L22 (1–3 μg) or Flag-USP4 (0.5–2 μg), and 6myc-HAS2 (2 μg), and denaturated. Samples were subjected to immunoprecipitation with a myc antibody followed by immunoblotting with myc and P4D1 antibodies. A representative experiment out of two performed with similar results, and their quantification, is shown.