Figure 3.
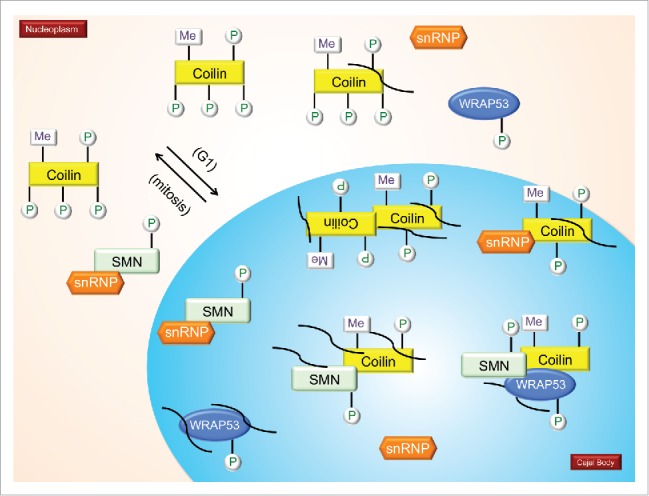
The impact of PTMs on the interactions and localizations of SMN and coilin. It is hypothesized that coilin in the nucleoplasm is hyperphosphorylated compared to coilin in CBs. During mitosis, coilin is hyperphosphorylated and CBs disassemble. CBs reform early to mid G1 (indicated in model). Mitotic coilin is reduced in its self-association compared to interphase coilin (paired coilin in the CB). Coilin interacts with SMN, Sm proteins, sRNPs and WRAP53. Methylation of coilin (and certain SM proteins) increases it interaction with SMN. Phosphorylation of coilin differentially affects its interaction with SMN and Sm proteins, with SMN preferentially binding to coilin that is less phosphorylated than that preferred by Sm proteins. The differential interaction of coilin with SMN and Sm proteins may disengage the nascent snRNP from the SMN complex in the CB, allowing for subsequent snRNP biogenesis steps, including snRNA modification and snRNP-specific protein association. Many non-coding RNAs, such as scaRNAs, snRNAs and snoRNAs are enriched in the CB and coilin complex (curved lines). Coilin interaction with RNAs decreases when coilin is hyperphosphorylated.12
