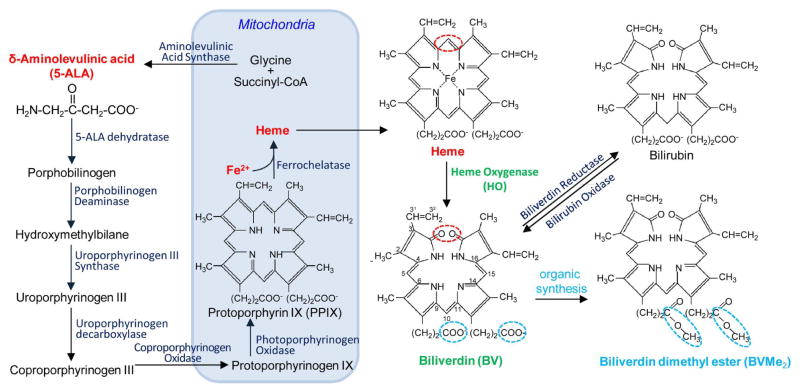
Figure 1. Scheme of biliverdin formation in mammalian cells.
Biosynthesis of a heme starts with the synthesis of 5-aminolevulinic acid (5-ALA) in mitochondria, and then 5-ALA is transferred to the cytosol. There it is merging in a chain of several enzymatic reactions through the formation of porphobilinogen (simple pyrrole), hydroxymethylbilane (linear tetrapyrrole) and uroporphyrinogen III (cyclic tetrapyrrole). Decarboxylation of uroporphyrinogen III results in a formation of coproporphyrinogen III, which is converted by coproporphyrinogen III oxidase, located in the mitochondrial membrane. Formed protoporphyrinogen IX is further oxidized to protoporphyrin IX (PPIX). PPIX is loaded by Fe2+ ions with help of ferrochelatase, which results in a formation of heme. Heme can be further converted to biliverdin IXα (BV) by heme oxygenase (HO), which cleaves the heme ring at the α-methene bridge (red ring in both heme and BV structures). In this reaction NADPH is used as the reducing agent. Molecular oxygen enter to the reaction resulted in carbon monoxide (CO) production, and the iron is released from the BV molecule. BV can be further converted into bilirubin with biliverdin reductase. Biliverdin dimethyl ester (BVMe2) can be produced using organic synthesis by esterification of carboxylic groups of BV (blue rings in BV and BVMe2 structures).