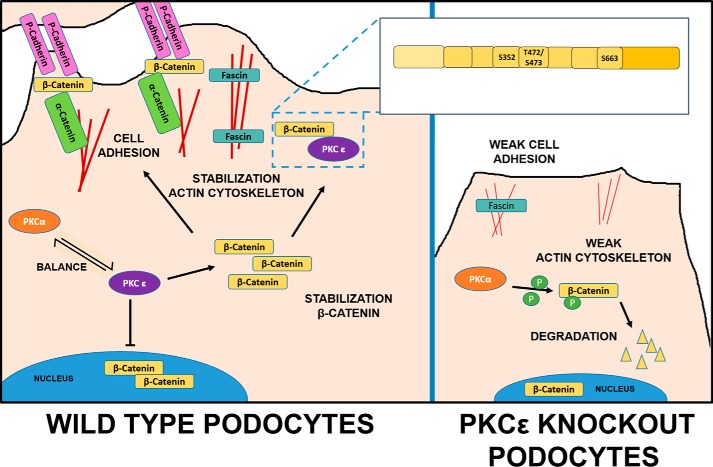
Figure 6.
Schematic overview of β-catenin regulation by PKCϵ in wild-type and PKCϵ−/− podocytes. In wild-type podocytes, PKCϵ regulates cytoplasmic β-catenin level in balance with other PKC isoforms: PKCα phosphorylates β-catenin and, thus, leads to its degradation. PKCϵ inhibits degradation via its interaction sites (Ser-352, Thr-472/Ser-473, and Ser-663) in a GSK3β-independent manner and leads to a predominantly cytosolic localization of β-catenin, which then might bind to the actin-bundling protein fascin1 in filopodia of the podocytes or localize to the membrane, forming the cell adhesion complex with P-cadherin and α-catenin, resulting in a stabilized actin cytoskeleton and cell adhesion. In PKCϵ-deficient podocytes, this balance is disturbed, leading to degradation of β-catenin, abolished P-cadherin expression, and lower fascin1 expression, which then results in impaired cell adhesion and actin bundling and, thus, in a weakened actin cytoskeleton.