Figure 2.
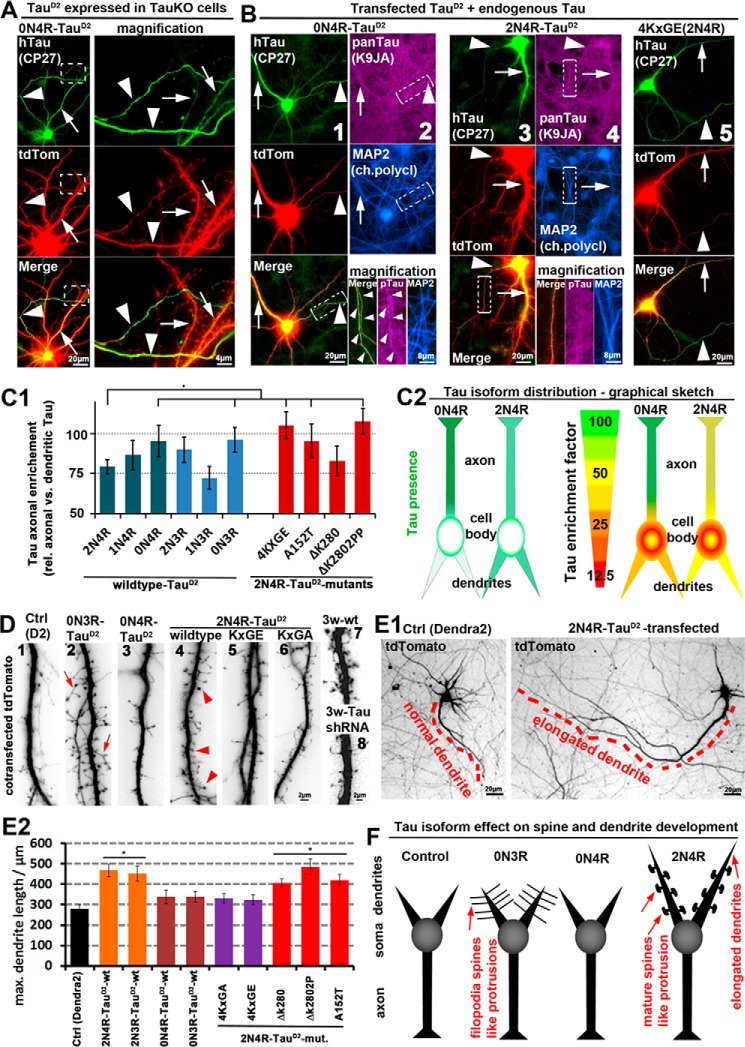
2N4R-Tau is retained in the somatodendritic compartment in wild-type neurons, where it induces accelerated spine and dendrite growth, whereas 0N4R-Tau can enter axons. Different isoforms of TauD2 were cotransfected with tdTomato (volume marker) for 6 days into primary neurons (7 DIV) derived from TauKO or wild-type mice. A and B, TauD2 transfected into TauKO cells (A) and wild-type cells (B). Transfected Tau was stained with an antibody against human Tau (CP27). Arrowheads, axon; arrows, dendrites. A, 0N4R-Tau shows strong enrichment in axons (for more constructs, see supplemental Fig. S1). B, wild-type neurons expressing endogenous Tau in addition to transfected exogenous TauD2 (stained with CP27). Exogenous and endogenous Tau are stained with a pan-Tau antibody (K9JA), and dendrites are stained with an antibody against MAP2. Columns 1 and 2, 0N4R-Tau is enriched in the axon. As a result, axonal Tau concentration is higher than in neighboring untransfected axons where only endogenous Tau is present, but it can barely be detected in dendrites with a pan-Tau antibody. Column 2 (bottom) shows magnified images from the boxed areas, and axon is indicated by arrowheads. Columns 3 and 4, 2N4R-Tau is not enriched in axons. Column 5, 2N4R-Tau pseudophosphorylated at the KXGS motifs (KXGS motifs mutated to KXGE) shows strong axonal enrichment. C1, quantification of axonal enrichment of different TauD2 isoforms and 2N4R-TauD2 mutations reveals lower axonal presence of the longest isoform of Tau (2N4R) compared with the other isoforms. The exception is the human-specific isoform 1N3R, not present in rodents. The mutations 4KXGE, A152T, and ΔK280PP of 2N4R-TauD2 increase the axonal sorting, whereas the mutation ΔK280 has no effect. C2, graphical sketch of the different distributions of the representative isoforms 0N4R (strong axonal enrichment) and 2N4R (weak axonal enrichment). Left panels, Tau distribution depicted in green. Right panels, Tau distribution in green presented relative to a volume marker with unbiased distribution in red, resulting in a ratiometric image. D and E, wild-type neurons with tdTomato as an unbiased volume/morphology marker. D, TauD2 transfection induces spine formation and maturation in young (10 DIV; 1–6) neurons, and Tau knockdown abolishes spine maturation in old (21 DIV; 7 and 8) neurons. 1, neurons only transfected with a control vector (Dendra2) show no spine formation and very few filopodia, representative of neurons on the brink of (but before) spine development. 2, transfection of 0N3R-Tau results in elongated filopodia-like protrusions up to 10 μm. 3, by contrast, 0N4R-Tau has no effect on dendritic spine/filopodia formation. 4, 2N4R-Tau-transfected cells show dendritic processes with mushroom heads (arrowheads), indicative of mature spines. 5 and 6, mutations of the KXGS motifs in the repeat domain to KXGE (5) or KXGA (6) of 2N4R-Tau prevent formation of protrusions and acceleration of spine maturation. For a comprehensive list of effects of all isoforms and several mutations, see supplemental Table S1. 7, aged control neurons display normal mature spines. 8, aged neurons transfected with shRNA against Tau display impaired spines. E1, Tau transfection induces dendritic outgrowth. Left, neuron transfected with a control vector (Dendra2). Right, neuron transfected with the 2N4R-Tau. The longest dendrites are indicated by red dashes. E2, quantification of the longest dendrite of cells transfected as indicated. F, graphical sketch of the dendritic effects of the representative Tau isoforms 0N3R (filopodia spines), 0N4R (no dendritic effects), and 2N4R (elongated dendrites and premature development of spines). *, p < 0.05. Error bars, S.E.