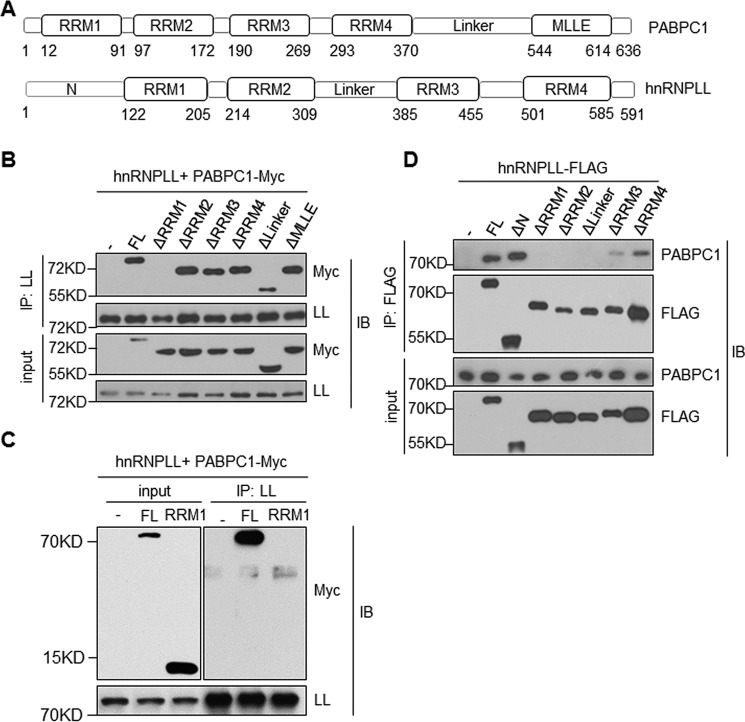
Figure 2.
Domain requirement for the hnRNPLL and PABPC1 interaction. A, a diagram shows domain structures of PABPC1 (upper panel) and hnRNPLL (lower panel). The location of each domain was indicated at the bottom. B, RRM1 domain of PABPC1 is required for its interaction with hnRNPLL (LL). HEK 293 T cells were transfected with indicated mutants of PABPC1 together with hnRNPLL. hnRNPLL was immunoprecipitated (IP), and its interaction with PABPC1 was analyzed by immunoblotting (IB). 1% of total protein lysates was used as an input control and analyzed for PABPC1 and hnRNPLL. C, the RRM1 domain of PABPC1 alone is not sufficient for interacting with hnRNPLL. HEK 293 T cells were transfected with Myc-tagged full-length or the RRM1 domain of PABPC1 together with hnRNPLL, respectively. hnRNPLL was immunoprecipitated with anti-mouse hnRNPLL antibody, and the immunoprecipitate was analyzed by immunoblotting. 1% of total protein lysates were used as input control. D, RRM1, RRM2, Linker, and RRM3 domains of hnRNPLL are required for its interaction with PABPC1. As in B, HEK 293 T cells were transfected with plasmids expressing FLAG-tagged hnRNPLL proteins lacking the indicated domains. The FLAG-tagged hnRNPLL proteins were immunoprecipitated with anti-FLAG, and their interactions with PABPC1 were determined by immunoblotting analysis. The data are representative of at least three independent experiments.