Figure 1.
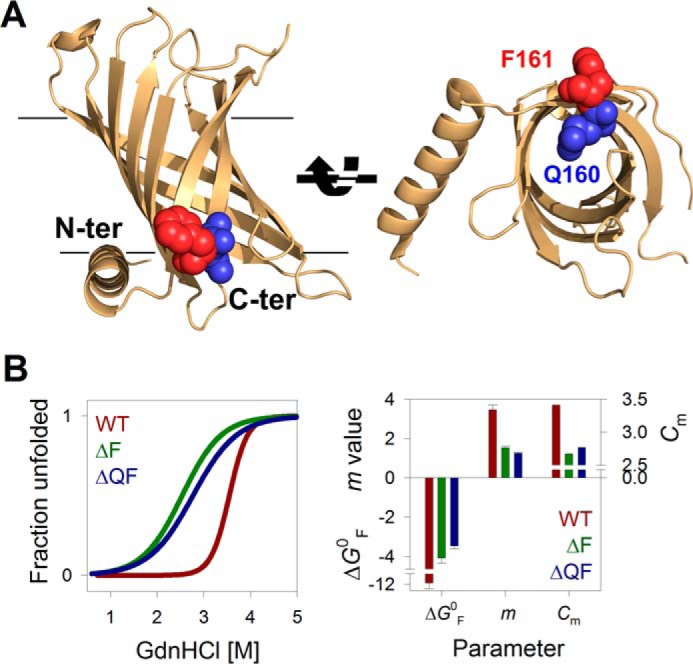
C-terminal residues affect PagP equilibrium folding free energy. A, schematic representations of E. coli PagP (PDB entry 3GP6) highlighting the C-terminal (Phe161; maroon) and penultimate (Gln160; blue) residues belonging to the β-signal of the 8-stranded transmembrane barrel. The structure on the right highlights how the Phe161 side chain projects toward the lipid environment, whereas that of Gln160 faces the protein interior. B, two-state equilibrium folding data of PagP-WT (WT; brown) and C-terminal deletion mutants PagP-ΔF161 (ΔF; green) and PagP-ΔQ160F161 (ΔQF; blue) obtained from GdnHCl-mediated chemical denaturation. Fluorescence intensities at the emission maximum were plotted as unfolded fraction from 0 to 1 and fitted to a two-state unfolding equation (fits are shown in the left panel; see supplemental Fig. S3 for the data) to derive the thermodynamic parameters ΔG0, m value, and Cm (right). Error bars, goodness of fit.
