Abstract
Non-standard exception requests (NSERs), for which transplant centers provide patient-specific narratives to support a higher MELD/PELD score, are made for > 30% of pediatric liver transplant candidates. We describe the justifications used in pediatric NSER narratives 2009–2014 and identify justifications associated with NSER denial, waitlist mortality, and transplant. Using UNOS data, 1,272 NSER narratives from 1,138 children with NSERs were coded for analysis. The most common NSER justifications were failure-to-thrive (48%) and risk of death (40%); both associated with approval. Varices, involvement of another organ, impaired quality of life, and encephalopathy were justifications used more often in denied NSERs. 60% of the 25 most prevalent justifications were not associated with approval nor denial. Waitlist mortality risk was increased when fluid overload or “post-transplant complication outside standard criteria” were cited, and decreased when liver-related infection was noted. Transplant probability was increased when the narrative mentioned liver-related infections, and fluid overload for children < 2 years old; it decreased when “post-transplant complications outside standard criteria” and primary sclerosing cholangitis were cited. This analysis provides novel insight and suggests targets for future consideration in outcomes research and exception criteria. Changes in the allocation system are needed to ensure equity and optimize outcomes for all pediatric candidates.
INTRODUCTION
The Model for End-Stage Liver Disease (MELD) and Pediatric End-Stage Liver Disease (PELD) scoring systems were implemented in 2002 to prioritize liver transplant candidates based on “objective and measurable medical criteria”. The system was designed to minimize waitlist mortality.(1) But even data from two years after MELD/PELD implementation suggested that the scores might not adequately describe transplant urgency for pediatric liver transplant candidates.(2) Since then, more than half of listed children have been granted “exceptions” to increase their MELD/PELD score and expedite transplant.(2,3)
In addition to standardized exceptions, e.g. for hepatocellular carcinoma or urea cycle disorders, non-standard exception requests (NSERs) have been highly utilized—in more than 40% of pediatric liver transplant candidates from 2009–2014 that are listed by calculated MELD/PELD. More than 90% of NSERs are approved Compared to children with no exception requested, those with an approved NSER have higher odds of transplant and reduced risk of post-transplant death after adjusting for other factors.(3,4) Despite the low prevalence of NSER denial, those who are denied have a lower likelihood of transplant, increased risk of waitlist mortality and, for those who are eventually transplanted, increased risk of post-transplant death compared to those with no NSER or approved NSERs. (3,4)
Each NSER is based on a narrative and request for a specific MELD/PELD score, submitted by the transplant center to the Regional Review Board (RRB). Per United Network for Organ Sharing (UNOS) policy, NSER applications must “justify why accepted medical criteria support that the candidate has a higher MELD/PELD score” and “explain how the patient’s current condition and potential for benefit would be comparable to that of other[s]” with that score.(5) The narrative summarizes the patient, their illness, their complications, and justifications for NSER. But there is no formal guidance on what constitutes “accepted medical criteria” or when to submit an NSER for pediatric candidates.
RRB composition and specific procedures vary by region. In some regions, all transplant centers are represented on the RRB (e.g. Region 6); in others (e.g. Region 5), voting membership rotates—with 7 serving at any given time. RRB members—which includes transplant physicians and surgeons from centers in the region—are alerted to an NSER by email and have 21 days to vote on the case. A brief comment may be submitted by RRB members who deny an NSER; these are provided to the submitting center. Transplant centers can appeal a denial, either in written form or by requesting a conference call discussion with the RRB. But the narratives have not otherwise been available for review and are not included in the standard-release Scientific Registry of Transplant Recipients (SRTR) database.
This is the first analysis of UNOS NSER narratives that focuses on pediatric liver transplant waitlist candidates. Our aims were (1) to describe the justifications used in NSER narratives and (2) to investigate which justifications are associated with NSER denial, waitlist mortality, and transplant. We utilized content analysis, in which qualitative data is coded and analyzed quantitatively, to code the narrative justifications. (6,7)
METHODS
Cohort Selection
We utilized the SRTR, which includes data on all waitlisted candidates in the United States, as submitted by Organ Procurement and Transplant Network (OPTN) member centers. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. Institutional Review Board approval from the University of California, San Francisco, was obtained prior to analysis (CHR 14-15024).
Included were pediatric liver transplant waitlist candidates, ages 0–18 years at listing, listed between 1 January 2009 and 31 December 2014. To focus on the impact of NSER justifications within the group that had NSERs, we excluded waitlist candidates with no exception requests and those who were initially listed as Status 1a, 1b, inactive, or with a “standard” exception that earned automatic MELD/PELD exception points. “Standard” exceptions included HCC within criteria, metabolic liver disease, hepatoblastoma, primary hyperoxaluria, hepatopulmonary syndrome or portopulonary hypertension, familial amyloidosis. We classified liver transplant indication based on the categories defined by the Studies in Pediatric Liver Transplant (SPLIT) Research Group.(8) Center volume was calculated as mean pediatric liver transplants per year, using annual data from 2009 through 2014.(9)
Narrative Analysis
The narratives submitted with each NSER were obtained from OPTN, after HRSA approval. For each waitlist candidate, we analyzed narratives accompanying the first approved NSER and all denials before the first approval, or all denials for those with no approvals. All narratives were read and coded by two authors (HB, EP) using a content analysis approach.(6,7) Prior to beginning review of the narratives, the author group generated an a priori list of likely NSER justifications based on our own experience running a pediatric transplant program and our review of literature on factors associated with transplant and waitlist mortality in pediatric liver transplant candidates. We added to the list of justifications during narrative review as needed to cover all themes. Each justification was coded as present or absent for each narrative. Narratives for which one reviewer was uncertain was reviewed by the other reviewer, and a random sample of narratives were cross-reviewed to ensure consistency in coding. Justifications were grouped to allow for summary by main themes, as demonstrated in TABLE S1.
Statistical analysis
Continuous variables were reported as mean ± standard deviation, with p-value by ANOVA, if normally distributed with equal variances. For skewed distribution and/or unequal variances (Bartlett’s test p<0.05), median (interquartile range), with Kruskal-Wallis testing, was reported. Categorical variables, including NSER justifications, were compared with chi-squared testing.
Only data from a patient’s first listing within the study period was used for outcomes analysis. For the NSER justifications and NSER-specific data, data from the first NSER within that listing was used.
Factors associated with NSER denial were evaluated using logistic regression. Competing risks regression was used to evaluate association of predictors with (1) liver transplant and (2) waitlist mortality, defined as a death or waitlist removal for being too sick to transplant.(10) NSER justifications mentioned in ≥5% of all NSERs were considered in univariate analysis, as were patient characteristics. Variables with p<0.20 i were tested in multivariable models, and factors were eliminated using backward stepwise selection. Because we identified an interaction between age and waitlist mortality in previous analysis, we evaluated each variable for interaction with age; interactions with p<0.05 were retained. Observation time was measured from the date of listing for transplant to waitlist death, liver transplant, or last date on the waiting list for patients still waiting or removed for other reasons.
To assess whether NSER justifications had an impact on approval after an initial denial, McNemar’s chi-squared test for paired data was utilized. SAS version 9.4 (SAS Institute, Cary, NC) or Stata/IC 14 (StataCorp, College Station, TX) were used for analyses.
RESULTS
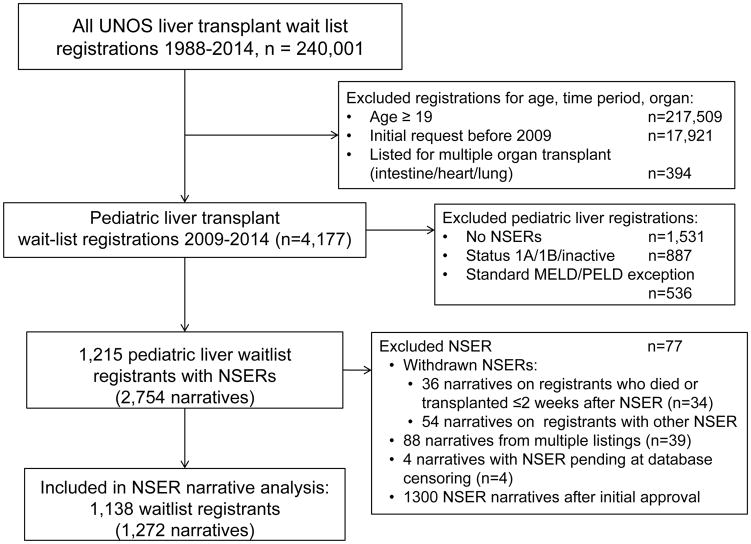
After initial exclusions, our cohort included 1,215 children who had NSERs. After additional exclusions, 1,272 NSER narratives on 1,138 pediatric liver transplant candidates were coded. (FIGURE 1) Of those children with NSERs, 82% had only approved NSERs, and 5% had an initial approval with later denial—these participants were combined in the NSER approval group. Nine percent had initial NSER denial with eventual approval, and only 4% of children had denials without approvals.
FIGURE 1.
Creation of the study cohort using UNOS SRTR files. MELD Mayo End-stage Liver Disease, NSER Non-standard Exception Request, PELD Pediatric End-stage Liver Disease, UNOS United Network for Organ Sharing.
Subjects with initial NSER denial were older, less likely to have biliary atresia, and less likely to have public insurance. Although those with initial NSER denial had higher listing MELD/PELD and 70% eventually had an NSER approved, they spent significantly longer on the waiting list and had significantly lower allocated MELD/PELD at transplant than those with initial NSER approval. For those transplanted, donor type did not differ by NSER status. (TABLE 1)
Table 1.
Clinical and transplant characteristics of liver transplant recipients, by initial NSER status*
| 1st NSER approved (n=997) | 1st NSER denied (n=141) | p* | |
|---|---|---|---|
| Female | 54.3% | 63.1% | 0.05 |
| Age at listing | 4.7 ± 5.7 | 10.8 ± 6.6 | 0.02 |
| ≤ 2 years | 55.3% | 19.9% | <0.001 |
| 2–12 years | 29.2% | 23.4% | |
| >12–18 years | 15.6% | 56.7% | |
| Weight (kg) at listing: median, IQR | 11.3 (6.7–28.1) | 40.8 (15.9–61.9) | <0.001 |
| Previous transplant (n=875) ¶ | 8.0% | 6.2% | 0.56 |
| Concurrently listed for kidney or pancreas | 3.8% | 9.9% | 0.001 |
| Ethnicity | |||
| White | 54.5% | 65.3% | 0.06 |
| Black | 17.4% | 9.2% | |
| Hispanic | 20.0% | 19.9% | |
| Asian | 5.2% | 4.3% | |
| Other | 3.0% | 1.4% | |
| Indication for liver transplant† | |||
| Biliary atresia | 49.7% | 25.5% | <0.001 |
| Cholestatic conditions | 11.9% | 19.2% | |
| Metabolic liver disease | 10.9% | 10.6% | |
| Tumor (outside standard criteria) | 5.4% | 8.5% | |
| Acute liver failure | 1.7% | 5.7% | |
| Other liver disease | 20.4% | 30.5% | |
| Public Insurance | 49.7% | 39.7% | 0.03 |
| Calculated MELD/PELD score at listing | 9 (2–16) | 11 (6–16) | 0.04 |
| Labs at listing | |||
| Creatinine (mg/dL) | 0.3 (0.2–0.47) | 0.52 (0.4–0.7) | <0.001 |
| Sodium | 137.2 ± 3.8 | 137.7 ± 3.55 | 0.11 |
| Albumin | 3.28 ± 0.73 | 3.23 ± 0.77 | 0.46 |
| INR | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) | 0.91 |
| Bilirubin | 7.62 ± 7.98 | 5.92 ± 7.70 | 0.02 |
| Lab MELD/PELD score at 1st NSER | 10.1 ± 10.3 | 11.8 ± 9.8 | 0.06 |
| Requested MELD/PELD at 1st NSER | 29.0 ± 8.7 | 29.0 ± 5.7 | 0.95 |
| Center volume (mean number of pediatric liver transplants annually at listing center, 2009–2014) | |||
| <5 | 11.5% | 9.9% | 0.66 |
| 5–15 | 32.3% | 35.5% | |
| >15 | 49.0% | 49.6% | |
| Missing | 7.2% | 5.0% | |
| Days from listing to 1st NSER | 16 (2–70) | 27 (6–82) | 0.03 |
| Total days on waitlist* (n=1032) | 105 (47–210) | 148 (88–364) | <0.001 |
| Outcome of 1st listing during study period (n=1,032) | |||
| Transplanted | 90.6% | 79.7% | ‡ |
| Died/too sick | 5.5% | 8.6% | |
| Improved/lost to follow-up | 3.9% | 11.7% | |
| Lab MELD/PELD at waitlist removal (n=1,010) | 12.8 ± 12.3 | 14.6 ± 11.6 | 0.13 |
| Allocation MELD/PELD at waitlist removal (n=907) | 31.6 ± 10.0 | 25.3 ± 8.8 | <0.001 |
| Medical condition at transplant (n=921) | |||
| Not hospitalized | 65.6% | 66.7% | 0.99 |
| Hospitalized, not ICU | 20.4% | 19.6% | |
| ICU | 8.6% | 7.8% | |
| Not known | 5.5% | 5.9% | |
| Transplant Type (n=921) | |||
| Living donor | 8.1% | 8.8% | 0.95 |
| Cadaveric donor, whole | 70.9% | 70.6% | |
| Cadaveric donor, split | 16.1% | 14.7% | |
| Donor deceased after cardiac death (n=921) | 0.12% | 0% | 0.86 |
| Donor CDC high-risk (n=799) | 6.3% | 4.6% | 0.53 |
| Cold ischemia time (n=858)* | 6.8 ± 3.7 | 7.2 ± 3.6 | 0.30 |
Continuous variables reported as mean ± SD with p-value by ANOVA for variables with normal distribution, equal variances, median (IQR) with p-value by Kruskal-Wallis test for variables with skewed distribution and/or unequal variances (Bartlett’s test p<0.05). When included, (n) in each row indicates number of patients for whom data on that variable was available in the SRTR database. Rows without n listed had no missing data for that variable.
UNOS only includes previous transplant indicator on listings that end in liver transplant.
Cholestatic conditions include Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis. Metabolic liver disease includes alpha-1-antitrypsin deficiency, Crigler-Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, and Wilson’s disease. Other liver disease includes congenital hepatic fibrosis, Budd-Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
See Results and Table 3, Supplemental Table 2 for statistical significance in analysis of waitlist mortality.
NSER narrative justifications and association with NSER approval or denial
We examined the prevalence of each coded justification in the first NSER narrative, and their association with initial NSER approval or denial. Justifications mentioned in more than 5% of coded NSER narratives are included in Table 2, and a full list is provided in TABLE S1. Specific complications of liver disease were cited in 86% of narratives, but overall the mention of any complication was not associated with NSER approval or denial. The most common complication was failure to thrive (FTT, 48% of all NSERs), and NSERs noting FTT were more likely to be approved. Explicit mention of “risk of death” was the second most common, and also associated with approval. However, most justifications were associated with neither approval nor denial. (TABLE 2, TABLE S1)
Table 2.
Justifications included in initial NSER narratives, prevalence and association with NSER denial*
| % of all NSER narratives with justification (n=1,138) | Odds of NSER denial | |||
|---|---|---|---|---|
| Univariate analysis | ||||
| OR | 95% CI | p | ||
| NSER justifications associated with approval | ||||
| Risk of Death | 39.4 | 0.57 | 0.39–0.84 | 0.005 |
| Failure to thrive, any mention | 47.8 | 0.56 | 0.39–0.81 | 0.002 |
| - Failure to thrive despite NG/GT or TPN | 28.4 | 0.38 | 0.23–0.62 | <0.001 |
| Biliary complications | 21.0 | 0.48 | 0.28–0.81 | 0.006 |
| NSER justifications associated with denial | ||||
| Varices (any mention, bleeding not specified) | 24.7 | 1.69 | 1.16–2.47 | 0.006 |
| Involvement of Other Organ System (any) ¶ | 19.3 | 1.79 | 1.20–2.68 | 0.004 |
| -Renal system | 5.9 | 2.83 | 1.60–5.02 | <0.001 |
| Impaired quality of life | 9.8 | 3.01 | 1.89–4.79 | <0.001 |
| Encephalopathy | 9.5 | 2.09 | 1.27–3.44 | 0.004 |
| Standard exception category, outside criteria for standard exception points | 34.1 | 1.51 | 1.06–2.16 | 0.02 |
| Justifications cited in ≥5% of NSER, not associated with approval or denial | ||||
| Complications of liver disease, any | 85.9 | 1.07 | 0.64–1.78 | 0.81 |
| Fluid overload (ascites, hydrothorax, anasarca) | 36.9 | 0.87 | 0.60–1.26 | 0.45 |
| Any liver-related infection | 34.1 | 0.83 | 0.57–1.21 | 0.34 |
| - Cholangitis | 25.0 | 0.79 | 0.51–1.21 | 0.27 |
| - Sepsis | 8.8 | 1.06 | 0.58–1.96 | 0.85 |
| Prolonged Hospitalization | 24.2 | 0.91 | 0.59–1.39 | 0.66 |
| Access to transplant limited by current status/score (any reason) | 18.8 | 1.08 | 0.69–1.68 | 0.73 |
| - No living donor options | 9.2 | 1.19 | 0.67–2.14 | 0.54 |
| Varices, with bleeding | 18.2 | 1.19 | 0.77–1.85 | 0.44 |
| Pruritus | 13.3 | 0.69 | 0.39–1.24 | 0.21 |
| Tumor (any, outside criteria for standard exception points) | 9.7 | 1.44 | 0.84–2.47 | 0.19 |
| Fat-soluble vitamin deficiencies | 9.3 | 0.63 | 0.32–1.28 | 0.20 |
| Post-transplant complication (any, outside criteria for standard exception points) | 8.6 | 0.99 | 0.52–1.85 | 0.96 |
| Involvement of Other Organ System: Bone ¶ | 5.8 | 1.62 | 0.85–3.12 | 0.15 |
| Involvement of Other Organ System: Pulmonary¶ | 5.5 | 0.73 | 0.31–1.73 | 0.48 |
Includes all justifications with ≥5% prevalence in initial NSER narratives. See Supplemental Table 1 for full listing, sub-categories, and prevalence of all justifications coded from the NSER narratives.
“Involvement” of other organ system includes organ disease or dysfunction caused by liver disease or as underlying/associated condition.
Use of some justifications was associated with increased odds of NSER denial. Mention of a diagnosis or complication that qualified for standard MELD/PELD exception points but for which the patient was outside of established criteria occurred in 34% of all NSER narratives, but was more commonly cited in denied than approved NSER narratives. Varices, involvement of another organ system, impaired quality of life, and encephalopathy were also mentioned significantly more often in denied NSERs. (TABLE 2, TABLE S2)
In multivariable analysis, NSER denial was associated with both impaired quality of life and involvement of the renal system. None of the NSER justifications remained significantly associated with NSER approval. Also associated with NSER denial were older age, higher MELD/PELD score at listing, and being listed in Region 5. (TABLE 3)
Table 3.
Multivariable association of NSER justifications, demographics, and patient location with outcomes*
| NSER denial | Waitlist mortality | Transplant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | |
| Demographics | |||||||||
| 2–18 years at listing (vs. ≤2 years) | 5.56 | 3.44–9.01 | <0.001 | 0.86 | 0.72–1.03 | 0.11 | |||
| Hispanic ethnicity (vs. any other) | 2.25 | 1.31–3.87 | 0.003 | ||||||
| MELD/PELD lab score (per 1 unit increase) | 1.03 | 1.02–1.06 | 0.002 | 1.04 | 1.01–1.07 | 0.02 | |||
| Public insurance | 0.82 | 0.71–0.94 | 0.005 | ||||||
| NSER justifications | |||||||||
| Fluid overload¶ | 1.78 | 1.04–3.06 | 0.04 | <2: 1.39 | 1.14–1.70 | 0.001 | |||
| 2–18: 0.97 | 0.79–1.20 | 0.80 | |||||||
| Any liver-related infection | 0.54 | 0.29–0.99 | 0.04 | 1.29 | 1.12–1.49 | 0.001 | |||
| Varices | 0.82 | 0.70–0.97 | 0.02 | ||||||
| Impaired quality of life | 1.97 | 1.15–3.38 | 0.01 | ||||||
| Involvement of other organ system: Renal | 1.96 | 1.02–3.80 | 0.05 | ||||||
| Post-transplant complication, outside standard criteria | 3.14 | 1.63–6.04 | 0.001 | 0.76 | 0.58–0.99 | 0.04 | |||
| PSC and/or autoimmune hepatitis¶ | † | † | † | <2: 1.02 | 0.73–1.44 | 0.90 | |||
| 2–18: 0.53 | 0.31–0.88 | 0.02 | |||||||
| Patient location | |||||||||
| Center volume (vs. 5–15 mean annual pediatric liver transplants at listing center, 2009–2014) | |||||||||
| <5 | 0.83 | 0.64–1.08 | 0.18 | ||||||
| ≥16 | 1.41 | 1.17–1.69 | <0.001 | ||||||
| Region (vs. Region 5) | |||||||||
| Region 1 | 0.08 | 0.02–0.30 | <0.001 | 1.23 | 0.83–1.82 | 0.30 | |||
| Region 2 | 0.23 | 0.13–0.42 | <0.001 | 0.75 | 0.59–0.95 | 0.02 | |||
| Region 3 | 0.04 | 0.01–0.12 | <0.001 | 1.44 | 1.12–1.87 | 0.005 | |||
| Region 4 | 0.29 | 0.16–0.55 | <0.001 | 0.67 | 0.51–0.86 | 0.002 | |||
| Region 6 | 0.06 | 0.01–0.43 | 0.006 | 1.02 | 0.69–1.52 | 0.91 | |||
| Region 7 | 0.30 | 0.14–0.64 | 0.002 | 1.01 | 0.74–1.39 | 0.94 | |||
| Region 8 | 0.10 | 0.03–0.27 | <0.001 | 1.16 | 0.83–1.62 | 0.38 | |||
| Region 9 | 0.12 | 0.05–0.30 | <0.001 | 1.46 | 1.04–2.05 | 0.03 | |||
| Region 10 | 0.32 | 0.16–0.66 | 0.001 | 0.82 | 0.61–1.12 | 0.22 | |||
| Region 11 | 0.03 | 0.004–0.28 | 0.001 | 1.38 | 0.91–2.07 | 0.13 | |||
Only significant variables with p<0.05 included in this table. All justifications with ≥5% prevalence in all initial NSER narratives considered for inclusion in multivariable analysis. See Supplemental Table 3 for full listing of ORs, HRs and statistical significance in univariate analysis.
Statistical interaction by age category, as noted in table. In the transplant model, interaction variable statistical significance: p=0.01 for interaction between fluid overload and age, p=0.04 for interaction between PSC/autoimmune hepatitis and age.
No waitlist deaths or removals for being too sick for transplant in the PSC/autoimmune hepatitis group.
Association of NSER justifications with waitlist mortality and transplant
Waitlist mortality risk was increased when fluid overload or post-transplant complications outside of standard exception criteria were included as NSER justifications (p<0.05, TABLE S3). Both factors retained significance in multivariable analysis. (TABLE 3) Decreased risk of waitlist mortality was identified for patients with narratives noting liver-related infection or cholangitis (TABLE S3, TABLE 3).
Probability of transplant was increased in univariate analysis for NSER justifications risk of death, fluid overload, and liver-related infection—specifically cholangitis. In multivariable analysis, increased probability of transplant remained associated with liver-related infection and fluid overload, the latter only in children < 2 years old at listing. Probability of transplant was decreased for patients with NSER narratives describing fat-soluble vitamin deficiencies, varices, impaired quality of life, encephalopathy, post-transplant complication outside of standard criteria, and PSC/AIH. In multivariable analysis, probability of transplant remained significantly reduced for patients with NSERs indicating varices, post-transplant complication outside standard criteria, and PSC/AIH in older children. (TABLE S3, TABLE 3)
The variable most strongly associated with increased risk of waitlist mortality and decreased likelihood of transplant was previous liver transplant, with complications that did not fit criteria for automatic exceptions. Of these 98 children, 20 (20%) had hepatic artery thrombosis outside of the 14-day window; none died on the waiting list. Eleven children (11%) died/ removed from waitlist; 4 had chronic rejection, 4 vascular insufficiency, 2 infected biliary strictures, and 1 autoimmune hepatitis. Nine were 7–18 years old at re-listing; 3 had denied NSERs and 8 approved. Of the 67 children re-transplanted, 7 patients died within one month post-transplant, after spending 23–895 days on the waiting list. Cause of death was graft dysfunction in 3, hemorrhage 2, and multi-organ failure/sepsis 2. Three died >6 months post-transplant.
Of 110 children whose narratives noted tumor outside standard criteria, 88 were transplanted. 7 died post-transplant (8%), all 6 months to 2 years post-transplant of disease recurrence; all had NSERs approved pre-transplant. The 92% of these children alive post-transplant had median follow-up 673 days, IQR 285–1437 days.
Regional variation in requested MELD/PELD scores
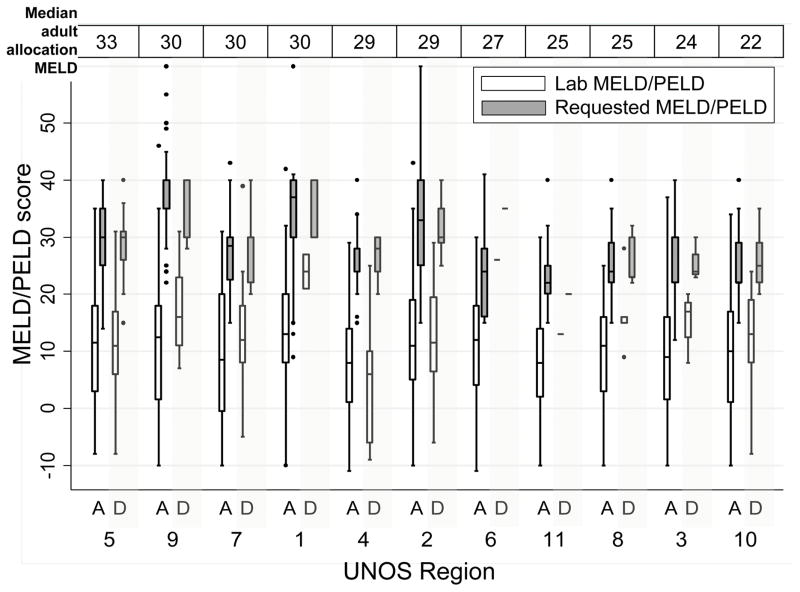
At 1st NSER, median calculated MELD/PELD was similar across regions, ranging from 8 in Region 4 (IQR 1–14) to 14.5 in Region 1 (IQR 8–20.5) (FIGURE 2) There were no statistically significant differences in calculated MELD/PELD at NSER or requested MELD/PELD between those with initial approval and initial denial in any region (p>0.05). (FIGURE 2, A vs. D for each region)
FIGURE 2.
Lab MELD/PELD score and requested MELD/PELD, at the time of first NSER, by region and NSER outcome (A = 1st NSER Approved, D = 1st NSER Denied). Boxes represent median and interquartile range (25th – 75th percentile) of MELD/PELD scores, whiskers mark the most extreme values within 1.5 x the interquartile range. Regions 6 and 11 had only one patient each with initial NSER denied.
Regions are ordered from highest to lowest median allocation MELD score for adult liver transplant recipients in the region, based on UNOS data from June 2012–June 2013 (OPTN Liver and Intestinal Organ Transplantation Committee Forum 9-16-2014, https://www.transplantpro.org/wp-content/uploads/sites/3/14-Share-35_Edwards.pdf, Accessed 12-10-2016.)
Changes in the NSER between denial and approval: MELD/PELD requests and narrative justifications
We next compared the approved and denied NSERs in patients that had both. Eighty-one patients had one NSER denial preceding an approval. Compared to those patients with only approvals (n=997), patients with one denial followed by approval were older (median at listing 13 years, IQR 4–16 vs. 1 year, IQR 0–9, for only-approvals, p<0.001) and less likely to have biliary atresia (19.7%, vs. 49.7%, p<0.001). Subjects with one denial before approval were more likely to be listed in region 5 (38%, vs. 9.4% of only-approvals) and region 10 (12.4%, vs. 6.7%, p<0.001 for all regions). There were no significant differences by calculated MELD/PELD at listing (median 9 for both, p=0.95) or at waitlist removal (median 11 for denial/approval, 12 for only-approvals, p=0.74), gender, race/ethnicity, or insurance type (data not shown). Outcomes were comparable, with 77.8% of the denial/approvals and 82.1% of the only-approvals getting transplanted, and 3.7% and 5.0%, respectively, dying on the waitlist or being removed for being too sick.
In these 81 patients, median requested score for the initial denial was 30 (IQR 24–30), and for the subsequent approval was 25 (IQR 22–30, p<0.001). Median decrease in requested points was 3 (IQR 0–7). Whether a justification was mentioned in the NSER did not change for most subjects between denial and approval (range 79.0% to 97.6% of narratives for each justification had no change, TABLE S4). The only justifications for which addition was associated with approval were sepsis (added to 4.9% of narratives, p=0.01), involvement of any organ system (added to 16%, p=0.03), and developmental delay worsened by liver disease (added to 7.4%, p=0.01). The most commonly added between denial and approval were complications of liver disease (added to 13.6% of narratives), FTT (9.9%), impact on quality of life (9.9%), and involvement of any organ system (16.0%), particularly of the renal system (7.4%).
NSER changes for those with multiple denials
Fourteen patients had two denials followed by approval. One with PTLD, cholangitis, and chronic rejection became too sick to transplant. Of the seven transplanted, four had narratives that described worsening complications of liver disease including ascites, FTT, variceal bleeding, and cholangitis. The fifth had HCC outside criteria, and 2 had no substantive changes to the narrative. Two that reached transplant had no changes in requested MELD/PELD between denial and approval (30 points requested), 3 had increased requests of 3–4 points, and 2 had decreases (23→21 and 28→22). All six that improved or were still waiting had decreases in their requested scores between denial and approval, of 2–10 MELD/PELD points.
Four patients had 3–4 denials preceding an approval. Three patients were teenagers, 12–18 years old. One with biliary atresia and HCC had 4 NSER denials before an approval; there were no changes in narrative during this process. A second who underwent initial liver transplant for ALF and developed biliary strictures with recurrent cholangitis as well as a PTLD-like process requiring bone marrow transplant had 3 denials followed by an approval. MELD request was decreased from 40 to 22; the patient remained listed at data censoring. A third adolescent underwent initial liver transplant for PSC, complicated by strictures and chronic infections. MELD request decreased from 40 to 22, and transplant occurred after more than 1 year on the waitlist. The fourth patient was a child with AIH and PSC who was approved after developing FTT despite supplementary feeding and biliary abscesses. This patient was recorded as a transfer to another center.
NSERs in patients with no approvals
There were 42 patients with only denials: 32 patients had 1 denial, 9 had 2 and 1 had 4. Of those 42 patients, 16.7% died or became too sick for transplant—compared to 5.0% of those with only approvals. Seventy-one percent of those with only denials were transplanted—compared to 82.2% of those with only approvals (p=0.005). Patients with only denials had higher median calculated MELD/PELD at listing (15.5, IQR 8–21, p<0.001) and at waitlist removal (19, IQR 10–26, p<0.001) but lower allocated MELD/PELD at transplant because of exception points (median 21, IQR 10–27, p<0.001) than those with only approvals (listing: 9, 2–16; waitlist removal: 11, 4–21 ; allocation 30, IQR 25–38). Twenty-six percent of those with only denials were in region 5, compared to 9.4% of those with only approvals (n=997, p=0.03).
The 32 patients with only denials were significantly older than those with only approvals (median 13.5 years, IQR 0.5–17 vs. median 1, IQR 0–9, p<0.001) but there was no difference in distribution of diagnoses (p=0.81, prevalence not shown). In their NSER narratives, children with only one denial were more likely to have hyponatremia (12.5% vs. 3%, p=0.01). encephalopathy (19% vs. 8.5%, p=0.05), sepsis (19% vs. 8%, p=0.05), impaired quality of life (22% vs. 8%, p=0.007), and involvement of another organ system (37.5% vs. 18%, p=0.005) than those with only approvals.
Among the 9 patients with 2 denials and no approvals, all were 10–18 years of age at listing. Two died or were removed for being too sick (22%). One recovered, and six were transplanted. Of the 5 that received deceased donor transplants, 4 had NSER narratives noting fluid overload, 3 of those also noted bleeding varices and risk of death.
DISCUSSION
This is the first analysis of NSER narratives to focus on pediatric liver transplant candidates since implementation of the MELD/PELD scoring system in 2002. It is intended describe what justifications are commonly used for pediatric NSERs, not prescribe what they should contain or which should be approved.
Although each narrative is personalized, we identified similar themes across most narratives. Many justifications commonly used in these NSER narratives are not factored into the MELD/PELD but are linked to life-threatening events. One other review of adult and pediatric NSERs, using UNOS 2005–2008 data, also identified ascites, infections, “wasting,” and varices as the most common justifications for pediatric NSERs.(11) In our analysis, NSER approval was associated with only three of the 25 most common justifications in univariate analysis. In multivariable analysis, the likelihood of NSER approval was associated with age at listing and region—but none of the NSER justifications. This suggests that NSER approval may be primarily based on factors outside of the narrative.
Patients with NSER denials followed by approvals had few changes in their NSER narratives. Most adjustment was in the requested score. This could reflect RRB opinion on the patient’s mortality risk—or opinion on other factors, like where the exception score would place patients relative to others on the waiting list. System efficiency might be increased by including a standardized list of justifications in NSER submissions—either to guide the narrative or as a checklist to replace it. Since most appeals involved a change in score request with little to no change in the narrative, we could also allow for adjustment of the requested score within the application or through RRB recommendation.
Several NSER justifications were more likely to be mentioned in denied than approved NSERs. Some of these justifications may not be compelling to RRBs. For example, the association of standard exception categories outside criteria with denial may reflect attempts to maintain system integrity. Others might be utilized when more convincing reasons are not applicable. For example, “impaired quality of life” and “encephalopathy” may be associated with denial because they were often described in patients that lacked directly life-threatening complications like bleeding varices or infections.
Comparing predictors of NSER approval/denial to those of waitlist mortality and transplant provides insight into what groups the current system may adequately advocatine for—and which are left vulnerable. Risk of death, FTT, and biliary complications were associated with NSER approval but not transplant or waitlist mortality. This may indicate that the NSER system appropriately factors these issues into waitlist priority. However, post-transplant complications outside standard exception criteria were associated with decreased probability of transplant and increased risk of death. These children appear to be at higher risk than currently accounted for in the system.
We found substantial regional variability in NSER approval rates, as has been reported previously.(3,11,12) Region remained a significant predictor of NSER approval and transplant, but not waitlist mortality, in this cohort. In previous analyses that included children without exception requests, region was not a significant predictor of transplant, waitlist mortality, or post-transplant death; NSER approval and denial did impact all three outcomes in that larger population.(3, 4) Listing at a low-volume center (<5 pediatric liver transplants annually) has also been associated with lower transplant probability and higher mortality risk.(13) Children listed at high-volume centers were more likely to be transplanted. Center volume was not associated with other outcomes. However, we did not examine whether children at low-volume centers were less likely to have NSERs filed.
Within each region, neither lab MELD/PELD scores at NSER nor requested MELD/PELD were significantly different between the NSER approved versus denied. Requested scores were generally higher in regions with higher median allocation MELD for adult liver transplant recipients—suggesting that transplant centers are calibrating their requests to “competition” for organs in the region. Potential behavioral changes in response to policy changes should be considered in planning system changes.
The high prevalence, and limited set, of common justifications supports that idea that more uniform and transparent NSER policies and practices could be generated by the pediatric liver transplant community. OPTN is planning to replace RRBs with National Review Boards, with a separate committee for pediatric candidates. (https://optn.transplant.hrsa.gov/governance/public-comment/national-liver-review-board/) The Pediatric National Review Board will include one member from each pediatric transplant center, and each NSER decision will be made by 5 randomly selected members of that Board. Our analysis provides some insight for Review Board members and transplant centers into common components of NSER narratives nationally.
A National Review Board system offers an important opportunity to equalize NSER practices across regions, but does not ensure transparency of the system or optimization of priority ranking. It should help ensure that decision-makers about pediatric NSERs have expertise about pediatric liver disease and mortality risk factors. Periodic published summaries of Review Board decisions would give transplant centers some additional insight into how the system operates.
Ongoing work to build the evidence basis on which NSER decisions are made and to standardize decisions is also crucial. The justifications associated with waitlist mortality and transplant could be targets for future research and for consideration in standardized exception criteria. For example, fluid overload was associated with increased transplant probability in younger but not older children, and with increased risk of waitlist mortality. Ascites has previously been associated with waitlist mortality in pediatric patients,(14) but recent UNOS guidance recommended against NSERs for ascites in adult candidates.(15)
Hyponatremia has been linked to waitlist mortality in pediatric patients, (14,17) and was recently added to MELD scores, but was rarely mentioned in the pediatric NSERs. For adults, serum sodium and creatinine may capture mortality risk associated with ascites. But this may not be accurate for adolescents—for whom creatinine is lower than in adults—and children—as neither sodium nor creatinine is in the PELD. Future research into how FTTcould be more accurately classified may also help with risk stratification. For example, a growth chart provided with NSERs could provide an objective, standardized picture of FTT severity and trajectory. Or an accompanying photograph could allow for an “eyeball test,” more formally termed subjective global assessment, of illness severity.(16)
Two recent documents from UNOS provide evidence-based guidance on common justifications for adult NSERs. Similar guidance for pediatric NSERs—which will require additional research to provide an robust evidence basis—could help standardize approvals for Review Board members.(15,18)
In this analysis, we reviewed the entire population of pediatric NSER narratives. This should minimize sampling bias. We used the narratives to deduce what transplant centers thought was important or effective, but we cannot discern how they decided which justifications to include. It is possible that we missed themes or coded justifications differently than other investigators might have done. Data on transplant offer acceptance/refusal was not available for this analysis; connecting that data to the NSER data would allow for additional—although still indirect—insight into transplant centers’ decision-making. Not captured in the UNOS database is direct information about what details influence reviewer decision-making. We also acknowledge that each individual’s story is complex and multi-faceted—we have not yet identified clusters of justifications that impact NSER approval or denial, or could act as a harbinger of dangerous outcomes.
In summary, this analysis suggests that the current NSER narrative system may not be efficient or effective for prioritizing pediatric liver transplant candidates by “objective and measurable” medical criteria. Particularly given the differential impact that NSERs appear to have on transplant and survival for older children, and by region for all children, UNOS should appoint a working group to focus on improvements to the MELD/PELD system as it applies to pediatric candidates. The NSER narratives do provide insight into how pediatric transplant centers justify NSERs and which justifications may or may not compel RRBs. To optimize equality and outcomes for pediatric liver transplant candidates, further work is required to build the evidence basis on which NSER decisions are made and to adjust policy so that we can decrease the need for NSERs.
Supplementary Material
Table S1: Justifications for initial non-standard exception requests (NSERs) in pediatric liver transplant candidates, including all coded justifications
Table S2: Association of clinical characteristics with NSER denial and approval*
Table S3: Univariate associations between demographics, NSER justifications, patient location and outcomes*
Table S4: Changes in the justifications included in NSER narratives of 81 children that had one NSER denial followed by NSER approval
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C (UNOS Data), the NIH-NIDDK (Dr. Perito, K23 DK0990253-A101), an American Society for Transplant Surgery-Alexion Presidential Student Mentor Grant (Ms. Braun), a UCSF Department of Pediatrics Clinical-Translational Pilot Grant (Dr. Perito), and the UCSF Liver Center (P30 DK026743). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, nor does mention of trades names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- CDC
Centers for Disease Control
- DCD
Deceased after cardiac death
- FTT
Failure to thrive
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- HRSA
Health Resources and Services Administration
- ICU
Intensive care unit
- INR
International normalized ratio
- IQR
Interquartile range
- MELD
Medical End-stage Liver Disease
- NSER
Non-standard exception request
- PELD
Pediatric End-stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- RRB
Regional Review Board
- SHR
Subhazard ratio
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4(Suppl 9):114–131. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 2.Shneider BL, Suchy FJ, Emre S. National and regional analysis of exceptions to the Pediatric End-Stage Liver Disease scoring system (2003–2004) Liver Transpl. 2006 Jan;12(1):40–45. doi: 10.1002/lt.20662. [DOI] [PubMed] [Google Scholar]
- 3.Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant. 2015 Feb;15(2):436–444. doi: 10.1111/ajt.13089. [DOI] [PubMed] [Google Scholar]
- 4.Braun HJ, Perito ER, Dodge JL, Rhee S, Roberts JP. Nonstandard Exception Requests Impact Outcomes for Pediatric Liver Transplant Candidates. Am J Transplant. 2016 May 23; doi: 10.1111/ajt.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network Policies. [Accessed 12 October 2016];Policy 9.3: Allocations of Livers and Liver-Intestines. Available at: https://optn.transplant.hrsa.gov/governance/policies/
- 6.Tong A, Chapman JR, Israni A, Gordon EJ, Craig JC. Qualitative research in organ transplantation: recent contributions to clinical care and policy. Am J Transplant. 2013 Jun;13(6):1390–1399. doi: 10.1111/ajt.12239. [DOI] [PubMed] [Google Scholar]
- 7.Tong A, Morton RL, Webster AC. How Qualitative Research Informs Clinical and Policy Decision Making in Transplantation: A Review. Transplantation. 2016 Sep;100(9):1997–2005. doi: 10.1097/TP.0000000000001358. [DOI] [PubMed] [Google Scholar]
- 8.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, et al. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a north american multicenter registry. Pediatrics. 2008 Dec;122(6):e1128–35. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed 31 August 2016];Organ Procurement and Transplantation Network Advanced Data Reports. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced.
- 10.Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496. [Google Scholar]
- 11.Gish RG, Wong RJ, Honerkamp-Smith G, Xu R, Osorio RW. United Network for Organ Sharing regional variations in appeal denial rates with non-standard Model for End-Stage Liver Disease/Pediatric End-Stage Liver Disease exceptions: support for a national review board. Clin Transplant. 2015 Jun;29(6):513–522. doi: 10.1111/ctr.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvalaggio PR, Neighbors K, Kelly S, Emerick KM, Iyer K, Superina RA, et al. Regional variation and use of exception letters for cadaveric liver allocation in children with chronic liver disease. Am J Transplant. 2005 Aug;5(8):1868–1874. doi: 10.1111/j.1600-6143.2005.00962.x. [DOI] [PubMed] [Google Scholar]
- 13.Rana A, Pallister Z, Halazun K, Cotton R, Guiteau J, Nalty CC, et al. Pediatric Liver Transplant Center Volume and the Likelihood of Transplantation. Pediatrics. 2015 Jul;136(1):e99–e107. doi: 10.1542/peds.2014-3016. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese R, Fonseca EA, Porta G, Danesi V, Guimaraes T, Porta A, et al. Ascites and serum sodium are markers of increased waiting list mortality in children with chronic liver failure. Hepatology. 2014 May;59(5):1964–1971. doi: 10.1002/hep.26776. [DOI] [PubMed] [Google Scholar]
- 15.OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. [Accessed 12 October 2016];Public Comment Proposal: Guidance to Liver Transplant Programs and the National Liver Review Board for Adult MELD Exception Review. https://optn.transplant.hrsa.gov/media/1923/liver_adult_meld_exception_guidance_20160815.pdf.
- 16.Englesbe MJ. Quantifying the eyeball test: sarcopenia, analytic morphomics, and liver transplantation. Liver Transpl. 2012 Oct;18(10):1136–1137. doi: 10.1002/lt.23510. [DOI] [PubMed] [Google Scholar]
- 17.Carey RG, Bucuvalas JC, Balistreri WF, Nick TG, Ryckman FR, Yazigi N. Hyponatremia increases mortality in pediatric patients listed for liver transplantation. Pediatr Transplant. 2010 Feb;14(1):115–120. doi: 10.1111/j.1399-3046.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed 12 October 2016];Guidance on MELD PELD exception review. Available at: https://optn.transplant.hrsa.gov/resources/by-organ/liver-intestine/guidance-on-meld-peld-exception-review.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Justifications for initial non-standard exception requests (NSERs) in pediatric liver transplant candidates, including all coded justifications
Table S2: Association of clinical characteristics with NSER denial and approval*
Table S3: Univariate associations between demographics, NSER justifications, patient location and outcomes*
Table S4: Changes in the justifications included in NSER narratives of 81 children that had one NSER denial followed by NSER approval