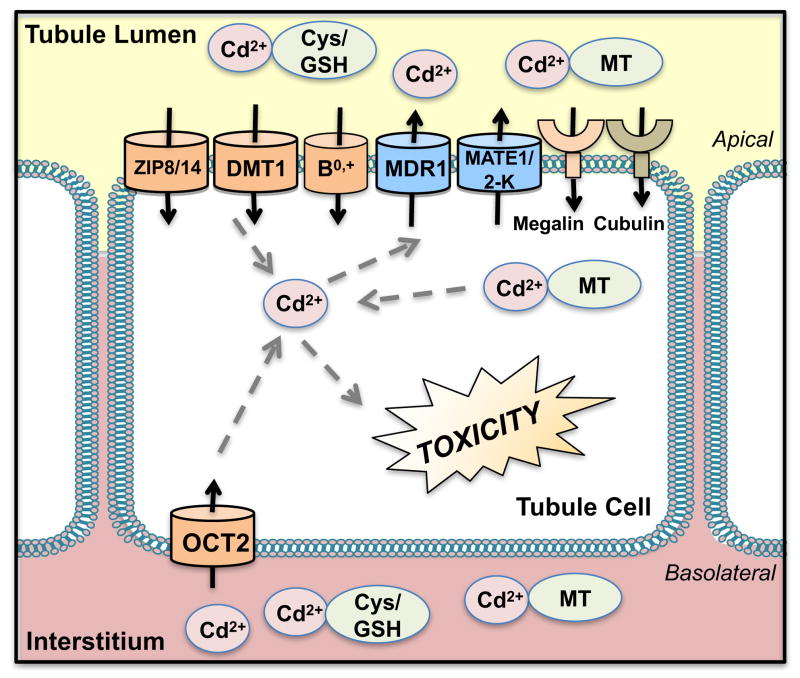
Figure 4. Transport of Cadmium.
Cadmium (Cd2+) is bound to albumin in the circulation after absorption. In the liver, Cd2+ forms a complex with metallothioneins (MT) that are released into the circulation. Cd2+ also binds to thiol-containing groups such as L-cysteine (Cys) and glutathione (GSH). After filtration, the Cd2+-MT complexes are reabsorbed by megalin and cubulin into the proximal tubules on the apical side and undergo degradation to ionic Cd2+. Cd2+ also displays “molecular mimicry” and uses divalent metal transporters (DMT1, divalent metal ion transporter-1) and zinc/iron proteins (ZIP8/14, zinc/iron-regulated transporter 8/14) for reabsorption. Amino acid transporters, such as system B0,+, may also reabsorb Cys-conjugates of Cd2+ on the apical plasma membrane. Organic cation transporter 2 (OCT2) (and possibly Oct1 in rodents) may also influx Cd2+ across the basolateral side of tubule cells. Multidrug resistance protein 1 (MDR1) and multidrug and toxin extrusion protein 1 and 2-K (MATE1/2-K) contribute to the limited Cd2+ that is secreted.