Abstract
To date, a recognized treatment for refractory membranous nephropathy (MN) has not been established. Recently, several reports have indicated the efficacy of rituximab as a novel treatment option. However, only a few published accounts exist of rituximab therapy for idiopathic MN (IMN) in the Asian population. We present the cases of three IMN patients who were treated with single-dose rituximab after they showed no response to conventional therapies, including corticosteroids, cyclosporine, cyclophosphamide, mizoribine, and mycophenolate mofetil. Although one case showed no response, a complete or incomplete remission was achieved in the other two cases. Rituximab may therefore be a beneficial treatment option for IMN.
Keywords: rituximab, membranous nephropathy, nephrotic syndrome
Introduction
Membranous nephropathy (MN) is a common cause of nephrotic syndrome (NS) in adults. Although several diseases such as viral infections, autoimmune diseases, certain drugs, and malignancies may cause secondary MN, most cases of MN are idiopathic MN (IMN) (1). Beck et al. identified the M-type phospholipase A2 receptor (PLA2R) as a major antigenic target in IMN (2). IMN is considered to be an organ-specific autoimmune disease. Previous studies report that approximately 50% of IMN patients with NS develop renal dysfunction; the ten-year renal survival rate is from 49% to 63% (3). Conventional therapies, consisting of corticosteroids and immunosuppressive agents, may be associated with significant adverse side effects and are not effective in all patients. Rituximab, a monoclonal antibody targeting the CD20 antigen of the B lymphocyte, has been reported to induce complete or partial remission (CR or PR) in patients with IMN since its first use in 2002 (4-10). To date, only a few cases of IMN have been treated with rituximab in Japan; thus, its efficacy in the Asian population has not been confirmed. In this case report, we present the cases of three adult Japanese patients with refractory IMN who were treated with rituximab.
Case Reports
Case 1
A 24-year-old man was admitted to another hospital because of newly developed peripheral edema. A urinalysis showed proteinuria with the excretion of 7.74 g/g creatinine, and laboratory tests revealed severe hypoproteinemia. The serum total protein and albumin levels were 3.9 g/dL and 0.9 g/dL, respectively. The patient was diagnosed with NS. The patient's renal function was normal (serum creatinine 0.94 mg/dL). He had been diagnosed with Stevens-Johnson syndrome nine years previously. His family history was otherwise unremarkable.
Immediately after hospitalization a renal biopsy was performed to obtain an accurate diagnosis. The sample contained 30 glomeruli, none of which showed global sclerosis. Light microscopy revealed a focal increase in mesangial matrix and mild mesangial hypercellularity in the glomeruli. Endocapillary cell proliferation was also detected in several glomeruli. The capillary walls displayed diffuse thickening. Immunofluorescence staining revealed high concentrations of IgG and C3 along the capillary walls. Staining for IgG subclasses revealed a strong IgG1 signal and weaker signals for IgG2 and IgG3; IgG4 staining was entirely negative. Electron microscopy revealed subepithelial deposits along the glomerular basement membrane (GBM). The renal pathological findings showed MN. The results of a serological workup for the hepatitis B surface antigen, hepatitis C antibody, anti-nuclear antibody, anti-neutrophil cytoplasmic antibody (ANCA), anti-GBM antibody, and rheumatoid factor were all negative. Serum immunoelectrophoresis showed no monoclonal proteins. Hypocomplementemia was not observed. A careful examination excluded the presence of malignant neoplasms.
Initially, the patient was treated with angiotensin II receptor blocker (ARB), anticoagulant, prednisolone (PSL; 60 mg/day), and cyclosporine (CyA; 150 mg/day). Six weeks later, the patient condition was still nephrotic. The treatment was then complemented by low-density lipoprotein apheresis (LDL-A). CyA was stopped, and intravenous cyclophosphamide (IVCY) therapy was subsequently started. The total amount of IVCY was 1,700 mg. However, after four months of treatment, no clinical or laboratory improvement was detected and the patient was transferred to our institution.
On admission, the patient had a blood pressure of 110/58 mmHg and a body weight of 55.4 kg. A physical examination revealed no edema of the limbs. A urinalysis showed proteinuria with the excretion of 7.34 g/g creatinine. The laboratory tests revealed a normal serum creatinine concentration of 0.69 mg/dL and a markedly decreased serum total protein concentration of 4.0 g/dL with a serum albumin concentration of 1.2 g/dL. Anti-PLA2R antibodies were not detected.
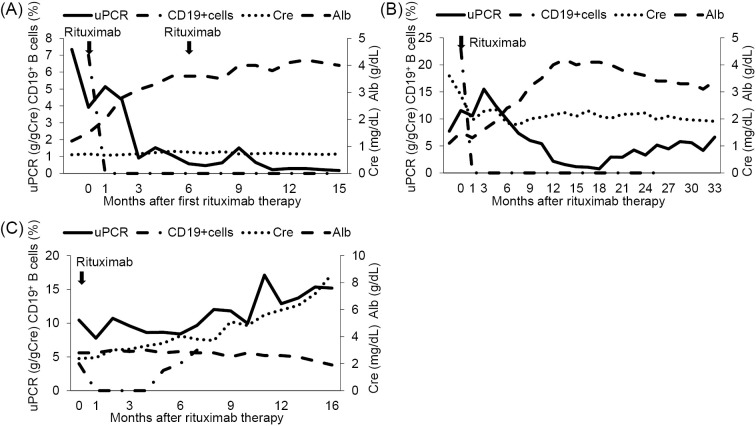
In view of his resistance to previous immunosuppressive therapies and his persistently severe nephrotic state, we administered single-dose rituximab (500 mg) at two weeks after his admission to our hospital. The premedication was diphenhydramine hydrochloride (30 mg) and acetaminophen (400 mg). The infusion was well tolerated. The PSL dosage was 25 mg/day and no immunosuppressive drugs were administered at the start of rituximab treatment. Six months after the injection of rituximab, the patient's urinary protein excretion was markedly reduced to 0.57 g/g creatinine with a serum albumin concentration of 3.6 g/dL. After the effectiveness of rituximab treatment was confirmed, a second 500 mg infusion of rituximab was administered. The levels of circulating CD19+ B cells were fully depleted from the time of the first rituximab injection to the present time (to date, the patient has been followed for 15 months). At the patient's last follow-up examination, urinary protein excretion was 0.17 g/g creatinine, the serum creatinine concentration was 0.72 mg/dL, and the serum albumin concentration was 4 g/dL. A CR was achieved and maintained (Figure). The PSL dose was gradually tapered to 3 mg/day and no immunosuppression agents were used. No adverse events were observed over the course of rituximab treatment.
Figure.
The clinical course of Cases 1 to 3. (A) Case 1. Single-dose rituximab (500 mg) was administered. Six months after the first injection, the same dose of rituximab was administered again. The patient’s urinary protein excretion was significantly decreased, and a complete remission was achieved at 11 months after the initiation of rituximab therapy. CD19+B cells were fully depleted throughout the observation period after the first rituximab treatment. (B) Case 2. Single-dose rituximab (630 mg) was administered. The patient’s urinary protein excretion gradually decreased. At one year after rituximab treatment, an incomplete remission was achieved. Although the patient’s urinary protein excretion increased again, nephrotic syndrome was not observed. CD19+B cells were fully depleted for 26 months after the administration of rituximab. (C) Case 3. Single-dose rituximab (600 mg) was administered. In this case, rituximab therapy was ineffective. The patient’s renal function progressively deteriorated, and peritoneal dialysis was initiated at 16 months after rituximab induction therapy. The CD19+B cells were fully depleted; however, they increased again at five months after the initiation of rituximab treatment. uPCR: urinary protein creatinine ratio, Cre: creatinine, Alb: albumin
Case 2
A 73-year-old man with a medical history of hypertension and hyperuricemia was admitted to another hospital for severe pedal edema and a weight gain of 5 kg. A urinalysis revealed the presence of massive protein and red blood cells in the urine. The urinary protein excretion was 10.40 g/g creatinine. The serum creatinine level was 1.74 mg/dL, and the serum total protein and albumin concentrations were 5.0 g/dL and 1.9 g/dL respectively. The patient was diagnosed with NS. His family history was otherwise unremarkable.
Two weeks after admission, a kidney biopsy was performed to make a histological diagnosis. Using light microscopy, 11 glomeruli were observed, one showed global sclerosis. Thickening of the GBM was observed. Mesangial proliferation, endocapillary hypercellularity, and extracapillary proliferation were not observed. Tubular atrophy and interstitial fibrosis were moderate with the patchy infiltration of mononuclear cells. Immunofluorescence staining revealed that the capillary walls were positive for IgG and C3. Staining for IgG subclasses showed intense IgG1 and IgG4 signals and a weaker IgG2 and IgG3 signals. The electron microscopic examination of the biopsy specimens revealed finely granular electron-dense materials along the GBM. The renal histological findings demonstrated MN. The results of serological tests for hepatitis B surface antigen, hepatitis C antibody, ANCA, anti-GBM antibody, and rheumatoid factor were all negative. The patient was positive for anti-nuclear antibodies; however, the presence of autoimmune disease was denied. Serum and urine immunoelectrophoresis showed no monoclonal bands. The serum complement titers were within the normal limits. Malignancy was excluded as the cause of secondary MN.
Initially, the patient was treated with PSL (15 mg/day) and CyA (75 mg/day). Eleven weeks later, his condition deteriorated. The serum creatinine levels elevated to 2.43 mg/dL, while his severe hypoalbuminemia showed no improvement (2.0 g/dL). High urinary protein excretion level was still observed (8.40 g/g creatinine). The patient was therefore referred to our hospital. At the first visit, his urinary protein excretion was 11.97 g/g creatinine. The laboratory investigations revealed a serum creatinine level of 2.34 mg/dL, a serum total protein level of 4.5 g/dL and a serum albumin level of 1.3 g/dL. He was admitted to our hospital and the PSL dosage was increased to 40 mg/day, with the addition of mizoribine (MZR; 100 mg/day). The MZR dosage was increased to 200 mg/day according to the blood concentration. However, these treatments were not effective. We therefore selected rituximab treatment for refractory IMN.
He was readmitted ten weeks after the first visit to our hospital. On admission, the patient had a blood pressure of 117/77 mmHg and a body weight of 64.0 kg. A physical examination revealed bilateral edema of the lower extremities. A urinalysis showed proteinuria with the excretion of 7.73 g/g creatinine. The laboratory tests revealed an elevated serum creatinine concentration of 3.59 mg/dL and a markedly decreased serum total protein level of 4.0 g/dL with a serum albumin level of 1.1 g/dL. No anti-PLA2R antibodies were detected. The administration of CyA and MZR was halted prior to the initiation of rituximab treatment. Only PSL was continued at a dose of 20 mg/day. Single-dose rituximab (375 mg/m2) was administered after premedication with diphenhydramine hydrochloride (30 mg) and acetaminophen (400 mg). The infusion was well tolerated. Several days after the injection of rituximab, the patient complained of odynophagia. The patient was diagnosed with an esophagus infection caused by herpes simplex virus and treatment with acyclovir was initiated. The circulating CD19+ B cells were fully depleted after the injection of rituximab. At 12 months after rituximab therapy, the patient's urinary protein excretion decreased to 2.16 g/g creatinine and an incomplete remission (ICR) II was achieved. At 18 months, the urinary protein excretion was significantly decreased to 0.75 g/g creatinine and an ICR I was achieved. The serum albumin level had markedly increased to 4.1 g/dL. To date, the patient has been followed for 33 months. The serum albumin levels have been maintained at over 3.0 g/dL and a quantitative analysis of the urinary protein showed that it had increased to 4-6 g/g creatinine. NS was not observed and the serum creatinine levels remained stable (range 1.91-2.18 mg/dL) (Figure). The dosage of PSL was gradually tapered to 4 mg/day and no immunosuppression agents were used.
Case 3
A 37-year-old man was admitted to our institution to undergo treatment for refractory NS. Twelve years previously, he had been admitted to another hospital for leg edema and an increase in body weight. His urinary protein excretion was 12.66 g/day. His serum creatinine level was 0.8 mg/dL, and his serum total protein and albumin levels were 3.5 g/dL and 1.7 g/dL, respectively. The patient was diagnosed with NS. His medical history was otherwise unremarkable. Interestingly, his father was also diagnosed with NS.
One month after his hospital admission, a kidney biopsy was performed. On light microscopy, ten glomeruli were observed, none of which showed global sclerosis. The capillary walls showed irregular thickening. Immunofluorescence staining revealed that the capillary walls were strongly positive for IgG and C3 and mildly positive for IgA, IgM, C4. Electron microscopy revealed subepithelial deposits. The histological diagnosis was MN. Tests for hepatitis B and C, anti-nuclear antibody and ANCA were all negative. Serum and urine monoclonal proteins were not detected. The serum complement titers were normal. Malignancy was excluded based on a clinical examination.
Although the patient was initially treated with angiotensin-converting enzyme inhibitor (ACE-I), PSL (60 mg/day) and CyA (200 mg/day), remission was not achieved. LDL-A was performed, but no therapeutic effect was confirmed. Despite the addition of MZR (150 mg/day), the nephrotic state continued. At 9 years after the onset of the disease, his renal dysfunction became apparent.
Thus, the patient consulted our hospital. At the first visit, his urinary protein excretion was 4.71 g/g creatinine. The laboratory investigations revealed a serum creatinine level of 1.46 mg/dL, a serum total protein level of 4.3 g/dL and a serum albumin level of 2.4 g/dL. MZR was stopped and the patient was switched to mycophenolate mofetil [MMF; 1,000 mg/day (temporarily, increased to 1,500 mg/day)], but the patient was unresponsive to the treatment. As a result of the unsuccessful treatment regimens, the patient was admitted for rituximab therapy at one year after the initial medical examination at our hospital.
On admission, the patient had a blood pressure of 148/92 mmHg and a body weight of 71.3 kg. A physical examination revealed bilateral pedal edema. A urinalysis showed proteinuria with the excretion of 10.44 g/g creatinine. The laboratory tests revealed an elevated serum creatinine concentration of 2.38 mg/dL and a decreased serum total protein level of 4.4 g/dL with a serum albumin level of 2.8 g/dL. Anti-PLA2R antibodies were detected. MMF was stopped prior to rituximab treatment. Only PSL (5 mg/day) was continued. Single-dose rituximab (375 mg/m2) was administered after premedication with diphenhydramine hydrochloride (30 mg) and acetaminophen (400 mg). The infusion was well tolerated. Despite rituximab therapy, nephrotic range proteinuria with hypoalbuminemia continued and the patient's renal function gradually deteriorated. On account of the ineffectiveness, PSL was also stopped. Sixteen months after the administration of rituximab, the patient presented with end-stage renal disease (Figure). Finally, peritoneal dialysis was initiated. No adverse events were observed over the course of rituximab treatment.
Discussion
We reported three cases of refractory IMN that were treated with rituximab. The baseline patient characteristics are shown in Table 1. Written informed consent was obtained from the patients for the administration of rituximab. The patients provided their informed consent for the publication of this case report.
Table 1.
Main Clinical and Laboratory Characteristics of Individual Patients at Baseline.
| Characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 24 | 73 | 37 |
| Gender (male/female) | male | male | male |
| Disease duration (months) | 4 | 6 | 150 |
| Clinical parameters | |||
| Body weight (kg) | 55.4 | 64.0 | 71.3 |
| Systolic blood pressure (mmHg) | 110 | 117 | 148 |
| Diastolic blood pressure (mmHg) | 58 | 77 | 92 |
| Laboratory parameters | |||
| Total protein (g/dL) | 4.0 | 4.0 | 4.4 |
| Serum albumin (g/dL) | 1.2 | 1.1 | 2.8 |
| Serum creatinine (mg/dL) | 0.69 | 3.59 | 2.38 |
| eGFR (mL/min/1.73m2) | 118.4 | 14.0 | 26.7 |
| Total cholesterol (mg/dL) | 259 | 369 | 246 |
| Urinary protein excretion (g/gCre) | 7.34 | 7.73 | 10.44 |
| anti-PLA2R antibody | negative | negative | positive |
| Treatment before rituximab | |||
| PSL (mg/day) | 25 | 20 | 5 |
| CyA (mg/day) | unused | 25 | unused |
| MMF (mg/day) | unused | unused | 1,000 |
| MZR (mg/day) | unused | 200 | unused |
| Former immunosuppressive drug | CyA, IVCY | nothing | CyA, MZR |
| RAS inhibitor agent | losartan 25mg | unused | unused |
eGFR: estimated glomerular filtration rate, Cre: creatinine, PLA2R: phospholipase A2 receptor, PSL: prednisolone, CyA: cyclosporine, IVCY: intravenous cyclophosphamide, MMF: mycophenolate mofetil, MZR: mizoribine, RAS: renin-angiotensin system
Recently, rituximab has been demonstrated as a potential treatment option for IMN. A summary of the clinical manifestations and therapeutic processes of previous representative reports is shown in Table 2. Remuzzi et al. treated eight patients who had IMN with persistent nephrotic syndrome (4). Six patients achieved a 50% reduction in their urinary protein excretion from baseline. In two patients, the proteinuria decreased to under 1 g/24 h at 20 weeks. This was the first report to provide evidence that rituximab treatment was associated with a significant reduction in urinary protein excretion in IMN patients. Thereafter, Ruggenenti et al. evaluated the one year outcome (5). Fervenza et al. conducted a prospective pilot trial of rituximab treatment in 15 patients with refractory IMN (6). Within the 12-month observation period, a CR was achieved in two patients and a PR was achieved in six patients. Furthermore, Fervenza et al. conducted a 2-year study of rituximab therapy for IMN (7). By the end of 24 months, a CR was achieved in four patients and a PR was achieved in 12 patients. Ruggenenti et al. described the treatment of 100 consecutive IMN patients with persistent nephrotic syndrome with rituximab (8). During a median follow-up period of 29 months after the administration of rituximab, 65 patients achieved a CR or PR. The median time to remission was 7.1 months. The remission rates were equal in patients with or without a history of immunosuppressive treatment. Cravedi et al. compared first-line rituximab therapy and second-line therapy for IMN and indicated the efficacy of rituximab treatment for the patients who failed to respond to previous immunosuppression (9). During follow-up, two patients in the second-line therapy group and three reference patients achieved a CR and five patients in each cohort achieved a PR.
Table 2.
Treatment Courses and Outcomes of Rituximab Therapy for Idiopathic Membranous Nephropathy (Summary of Reported Articles).
| Reference | N | Age (year) | Clinical presentation | Treatment before rituximab | Proteinuria before rituximab (g/24h) | Serum creatinine before rituximab (mg/dL) | Rituximab treatment dose | Observation period | Complete remission (definition per study) | Partial remission (definition per study) |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 52 (range 24-75) | persistent NS | full-dose ACE-I for 29.7 months | 8.6 ± 1.5* | 1.4 ± 0.3* | 375 mg/m2 every 4 weeks | 20 weeks | 2/8 (UP ≤1g/24h) | 3/8 (UP >1g/24h and ≤3.5g/24h) |
| 5 | 8 | 52 (range 24-75) | persistent NS | full-dose ACE-I for 29.7 months | 8.6 ± 1.5* | 1.4 ± 0.3* | 375 mg/m2 every 4 weeks | 12 momths | 2/8 (UP ≤0.5g/24h) | 4/8 (UP ≤3.5g/24h or 50% reduction versus basal) |
| 10 | 12 | 57± 13* | persistent NS | ACE-I at least 6 months | 10.3 ± 8.9* | 1.4 ± 0.5* | 375 mg/m2×1 (n=11), 375 mg/m2×2 (n=1) B cell-driven protocol; When ≥5 B cells/mm3 were detected in the circulation after the first administration, patients received a second infusion. | 12 momths | 2/12 (UP <0.3g/24h) | 6/12 (UP <3g/24h with a >50% reduction) |
| 6 | 15 | 47± 8* | persistent NS | RAS-I at least 4 months (n=15), Steroids alone (n=2), Steroids+cytotoxic agents (n=2), Steroids+CyA (n=2), Steroids+MMF (n=1) | 13.0 ± 5.7* | 1.4 ± 0.5* | 1g×2, on days 1 and 15; repeated at 6 month if proteinuria>3 g/24h and CD19+B cells>15/μL | 12 momths | 2/15 (UP <0.3g/24h) | 6/15 (UP ≤3g/24h with a >50% reduction) |
| 9 | 11 | 48.6± 13.9* | persistent NS | RAS-I at least 6 months (n=11), Steroids alone (n=2), Steroids+alkylating agents (n=6), Steroids+CyA (n=2), CyA alone (n=1) | 10.9 (range 6.6-18.6) | 1.3 ± 0.5* | 375 mg/m2 every 4 weeks (n=5) 375 mg/m2×1 or 2 (n=6) B cell-driven protocol; When ≥5 B cells/mm3 were detected in the circulation after the first administration, patients received a second infusion. | 24 momths | 2/11 (UP <0.3g/24h) | 5/11 (UP <3.5g/24h with a >50% reduction) |
| 7 | 20 | 48.6± 12.9* | persistent NS | RAS-I at least 4 months (n=20), Steroids alone (n=1), CyA alone (n=3), Steroids+CyA (n=1), Steroids+CY followed by MMF (n=2), Steroids+CY followed by CyA (n=1), Steroids+CY followed by CyA followed by MMF (n=2), Steroids+CY followed by MMF followed by Tac (n=1) | 11.9 ± 4.9* | 1.5 ± 0.5* | 375 mg/m2 every 4 weeks; re-treated at month 6 regardless of their clinical status. | 24 momths | 4/20 (UP ≤0.3g/24h) | 12/20 (UP≤3.5 g/24h and a 50% reduction in peak proteinuria with serum albumin >3g/dL) |
| 8 | 100 | 51.5± 5.9* | persistent NS | full-dose ACE-I at least 6 months (n=100) 32 patients had been exposed to 55 courses of different immunosuppressive regimens (Steroids alone, CyA alone, Steroids+alkylating agents, Steroids+CyA, adrenocorticotrophic hormone) | 9.1 (range 5.8-12.8) | 1.2 (range 0.97-1.7) | 375 mg/m2 every 4 weeks (up to October 2005), 375 mg/m2×1 patients received a second rituximab infusion only when >5 circulating B cells per mm3 were detected the morning after completion of the first rituximab administration. | 29 momths (range 6-121) | 27/100 (UP <0.3g/24h) | 38/100 (UP <3g/24h with a >50% reduction) |
* Variables expressed as mean ± standard deviation, NS: nephrotic syndrome, UP: urinary protein, ACE-I: angiotensin-converting enzyme inhibitor, RAS-I: renin-angiotensin system inhibitor, CyA: cyclosporine, CY: cyclophosphamide, MMF: mycophenolate mofetil, Tac: tacrolimus
In this case report, three patients who did not respond to corticosteroid and immunosuppressive agents, such as CyA, cyclophosphamide (CY), MZR, and MMF, received rituximab treatment as a second-line therapy. The clinical course of each patient is presented in Table 3. The remission statuses are defined according to the criteria established by Japanese Society of Nephrology (11). A CR was defined as proteinuria <0.3 g/day. An ICR was defined as resolution of NS but continuing proteinuria, and was divided into two grades: ICR I: (urinary protein: <1.0 g/day) and ICR II (urinary protein: 1.0-3.5 g/day). No response (NR) was defined as the persistence of nephrotic-range proteinuria (≥3.5 g/day).
Table 3.
Clinical Course of 3 Cases with Rituximab Treatment for Idiopathic Membranous Nephropathy.
| Case | Dose of rituximab | Baseline uPCR(g/gCre) | 3 months uPCR(g/gCre) | 6 months uPCR(g/gCre) | 12 months uPCR(g/gCre) | 18 momths uPCR(g/gCre) | Last visit uPCR(g/gCre) | Follow-up months | Response to rituximab therapy | Maintenance treatment after rituximab |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 500 mg×2 | 7.34 | 0.91 | 0.57 | 0.29 | not reached | 0.17 | 15 | CR | PSL 3 mg |
| 2 | 630 mg×1 | 7.73 | 15.50 | 9.87 | 2.16 | 0.75 | 6.61 | 33 | ICR I | PSL 4 mg |
| 3 | 600 mg×1 | 10.44 | 9.59 | 8.39 | 12.88 | 14.30 | no data | 29 | NR | (-) |
uPCR: urinary protein creatinine ratio, PSL: prednisolone, CR: complete remission, ICR: incomplete remission, NR: no response
The effects of therapy should be evaluated by 24-hour urine collection. If the collection of 24-hour urine is impossible, the ratio of urinary protein and urinary creatinine (g/g creatinine) in a spot urine test can be used.
In Cases 1 and 2, rituximab treatment was effective in treating refractory IMN as a second-line therapy. The patient from the first case achieved and maintained a CR at 15 months following the infusion of rituximab. The patient from the second case achieved a transient ICR I and his proteinuria subsequently increased again. However, his serum albumin levels have been maintained at >3.0 g/dL and NS was not observed for a long time period. The patient's renal function improved (serum creatinine level of 3.59 mg/dL before rituximab treatment and 1.91 mg/dL at last visit). He had no edema and his dosage of PSL could be significantly decreased. For these reasons, rituximab therapy was considered to have been beneficial in the second case. Considering his age and the side effect of infection, rituximab treatment was not repeated.
Case 3 showed no response to a single infusion of rituximab. Cravedi et al. conducted a prospective matched-cohort study that compared the administration of single-dose rituximab (375 mg/m2) with the standard protocol of four weekly rituximab (375 mg/m2) doses in the treatment of nephrotic IMN (10). At 12 months, in the single-dose rituximab group, 2 of 12 patients achieved a CR and 6 achieved a PR. Similarly, in the standard protocol group, two of 24 patients achieved a CR and 14 achieved PR. The administration of single-dose rituximab was as effective as the standard four-dose therapy in achieving a remission from IMN.
The circulating CD20+ B cells were already fully depleted after the first administration of rituximab and remained below the normal range throughout the whole observation period. Single-dose rituximab treatment could avoid unnecessary re-exposure to rituximab and minimize the medical costs. In addition, a reduction of rituximab dose may limit the production of antichimeric antibodies, which increase the risk for infusion reactions and interrupt retreatment for disease reactivation.
For these reasons, we treated IMN with a single dose of rituximab. In 2009, there was a significant breakthrough regarding IMN. Beck et al. identified the M-type PLA2R as the primary antigen in IMN (2). Beck et al. performed an assay to detect the anti-PLA2R antibody in two distinct cohorts of IMN patients who were treated with rituximab (12). At baseline, 25 of 35 (71%) patients had the anti-PLA2R antibody. At 12 months after rituximab therapy, these antibodies decreased in 17 (68%) patients. In the patients who reacted serologically, 59% and 88% achieved a CR or PR at 12 and 24 months, respectively. A reduction of the anti-PLA2R antibody titer was associated with decreased urinary protein excretion and preceded changes in proteinuria. Ruggenenti et al. evaluated the correlation between the levels of anti-PLA2R antibodies and the clinical course of IMN patients who were treated with rituximab (13). Eighty-four of the 132 IMN patients achieved the combined end points (CR or PR) within a median follow-up of 30.8 months. At baseline, 101 patients were screened for anti-PLA2R antibodies. The autoantibody could be detected in 81 of the 101 patients. The proportion of patients who entered remission was comparable in the patients with or without detectable anti-PLA2R antibody at baseline. Within the cohort of the 81 participants with antibody levels that were detectable at baseline, the probability of attaining the combined end points was 81.5%, 59.3% and 37.0% in the patients with the lowest (14-86 RU/mL), middle (87-204 RU/mL) and highest (>204 RU/mL) tertiles of antibody titer, respectively. The titer levels of anti-PLA2R antibodies at baseline were associated with the clinical outcome.
The anti-PLA2R antibody level was measured in all of our cases. Two patients (Cases 1 and 2) who attained remission were negative for the antibody. Rituximab suppresses the production of autoantibodies by means of B lymphocyte depletion and affects autoimmunity in IMN. Prunotto et al. detected specific anti-aldose reductase and anti-manganese superoxide dismutase (SOD2) IgG4 in the serum of IMN patients (14). There is the possibility that these patients may possess another autoantibody aside from anti-PLA2R. On the other hand, in the patient from the third case, in whom anti-PLA2R antibodies were detected, no therapeutic effect of rituximab was observed and his renal function gradually deteriorated. Thus, the anti-PLA2R antibody titer at baseline might have been very high.
Ruggenenti et al. evaluated the relevance of renal histological findings to therapeutic response in IMN patients who received rituximab (15). Tubular atrophy and interstitial fibrosis were significantly associated with poor treatment efficacy. In our cases, the therapeutic effect of rituximab was dissimilar. There are several possible explanations for the differences among the three cases. Case 1 showed the best response to rituximab therapy. In Cases 2 and 3, the serum creatinine levels had already been elevated at the initiation of rituximab treatment, whereas the creatinine level was normal in Case 1, the renal function might have influenced the treatment outcome. In particular, the patient from Case 2 had a medical history of hypertension. The bilateral kidneys were atrophic; thus, the presence of nephrosclerosis was suggested. Another explanation is that disease duration was associated with the outcome. In Case 3, rituximab treatment was started at 12 years after the onset of disease. The progression of tubulointerstitial damage may produce poor outcomes; thus, the initiation of rituximab at an early stage of IMN is critical.
Bomback et al. conducted a systematic review assessing the effects of rituximab treatment for IMN (16). This systematic review showed that several studies had reported adverse effects. Most of these side effects were mild, transient, and thought to be a reaction to the infusion. A small number of bacterial or viral infections were noted after the rituximab administration. However, no serious cases were reported. Laryngospasm and lung neoplasms were described as serious adverse events. In a prospective cohort study (8), 11 serious adverse events, which included eight cardiovascular events and three cancers, were reported, and four patients died. No treatment-related events, such as infection and myelotoxicity, were observed. In the present study, one elderly patient developed a herpes simplex viral infection shortly after the infusion of rituximab.
IMN is thought to be associated with a more favorable prognosis in Japanese individuals than in Caucasians. Shiiki et al. reported the results of a retrospective investigation of the prognosis and risk factors for renal survival in Japanese IMN patients with NS (17). Seven hundred ninety-five of the 949 (83.8%) patients achieved a CR or ICR, and 154 (16.2%) patients had persistent NS during the average follow-up period of 83.3 months. The patients who received conventional immunosuppressive therapy showed a significant improvement in their renal survival rates. However, massive proteinuria was a significant risk factor for end-stage renal disease. What kind of IMN patients are suitable for rituximab therapy? Although we indicated the efficacy of rituximab as a second-line therapy for IMN patients with NS, it is not clear whether rituximab treatment is superior to conventional therapies as an initial therapy for IMN. Currently, multicenter randomized trials comparing rituximab therapy with conventional immunosuppressive therapy for the treatment of IMN are ongoing (18,19).
In summary, rituximab may prove to be a beneficial treatment option for Japanese IMN patients who are refractory to immunosuppressive agents. However, our report only included a small patient population and the follow-up periods were relatively short. In relation to rituximab therapy for IMN, the appropriate dose, the number of times that it should be administered, and the appropriate treatment period remain unclear. Furthermore, the safety of the drug and the long-term therapeutic effects are unknown. We expect that future investigations will disclose the long-term efficacy and safety of rituximab therapy for IMN.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors express our special appreciation to Dr Y. Sato, Dr A. Tanaka, Dr F. Kamiya for their efforts. We also thank excellent technical assistance to N. Asano, Y. Sawa and N. Suzuki. This work was supported by a Grant-in-Aid for Progressive Renal Diseases Research, Research on intractable disease, from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Beck LH Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int 77: 765-770, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Beck LH Jr, Bonegio RG, Lambeau G, et al. . M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11-21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis 46: 1012-1029, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet 360: 923-924, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Ruggenenti P, Chiurchiu C, Brusegan V, et al. . Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol 14: 1851-1857, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Fervenza FC, Cosio FG, Erickson SB, et al. . Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117-125, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Fervenza FC, Abraham RS, Erickson SB, et al. ; Mayo Nephrology Collaborative Group Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol 5: 2188-2198, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruggenenti P, Cravedi P, Chianca A, et al. . Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416-1425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cravedi P, Sghirlanzoni MC, Marasà M, Salerno A, Remuzzi G, Ruggenenti P. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol 33: 461-468, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2: 932-937, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Nishi S, Ubara Y, Utsunomiya Y, et al. . Evidence-based clinical practice guidelines for nephrotic syndrome 2014. Clin Exp Nephrol 20: 342-370, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beck LH Jr, Fervenza FC, Beck DM, et al. . Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543-1550, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruggenenti P, Debiec H, Ruggiero B, et al. . Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545-2558, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prunotto M, Carnevali ML, Candiano G, et al. . Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 21: 507-519, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruggenenti P, Chiurchiu C, Abbate M, et al. . Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol 1: 738-748, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol 4: 734-744, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiiki H, Saito T, Nishitani Y, et al. ; Research Group on Progressive Renal Diseases in Japan Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int 65: 1400-1407, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Fervenza FC, Canetta PA, Barbour SJ, et al. ; Mentor Consortium group A multicenter randomized controlled trial of rituximab versus cyclosporine in the treatment of idiopathic membranous nephropathy (MENTOR). Nephron 3: 159-168, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Rojas-Rivera J, Fernández-Juárez G, Ortiz A, et al. . A European multicentre and open-label controlled randomized trial to evaluate the efficacy of Sequential treatment with TAcrolimus-Rituximab versus steroids plus cyclophosphamide in patients with primary MEmbranous Nephropathy: the STARMEN study. Clin Kidney J 5: 503-510, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]