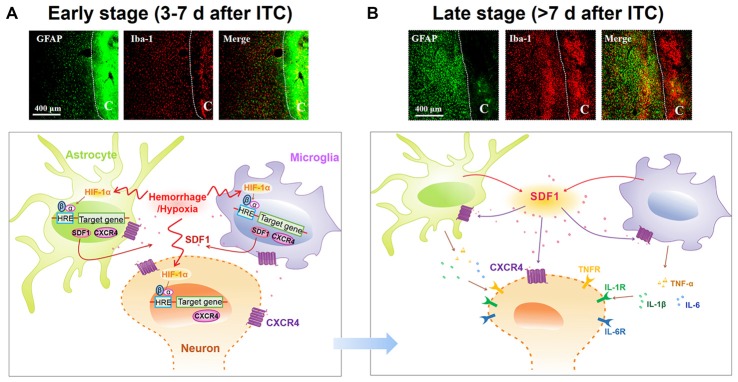
Figure 10.
Schematic illustration of microglial-astrocytic-neuronal interactions mediated by HIF-1α-SDF1-CXCR4 signaling following thalamic hemorrhage. (A) The upper panel shows representative immunofluorescent photomicrographs of microglial marker Iba-1 (red) and astrocytic marker GFAP (green) expression in thalamic sections at the early stage after hemorrhage (<7 days). The hemorrhagic lesion core is on the right side of the white line in each immunofluorescent image. At this phase, hypoxia microenvironment was established in peri-thalamic lesion site which induced accumulation of HIF-1α in the cytoplasm. As a transcription factor, HIF-1α translocates into the nucleus where it dimerizes with HIF-1β subunit and forms the active HIF-1 complex which binds to the hypoxia response element (HRE) in the DNA sequence and then induces CXCR4 expression in the neurons and glial cells as well as SDF1 expression in glial cells. The synthetic CXCR4 would integrate into cell membrane forming a functional receptor for neuron and glial cells while synthetic SDF1 would be secreted into extracellular space as a ligand (lower panel). Scale bar, 400 μm. (B) The upper panel shows representative immunofluorescent photomicrographs of microglial marker Iba-1(red) and astrocytic marker GFAP (green) expression in thalamic sections at the late stage after hemorrhage (>7 days). The hemorrhagic lesion core is on the right side of the white line in each immunofluorescent image. At this phase, abundant SDF1 from astrocytes and microglia activates CXCR4 on glial cells, resulting in the activation of glial cells by a positive feedback activation pattern and subsequent release of proinflammatory cytokines, including TNF-α, IL-1β and IL-6, which could subsequently increase neuronal excitation and glial activation in peri-thalamic lesion site. For the survival neurons in the peri-thalamic lesion site, the released SDF1 from glial cells could bind to CXCR4 and directly evoke neuronal hyperactivity which combined with glial activation contribute to the maintenance of CPSP (lower panel). Scale bar, 400 μm.