Figure 2.
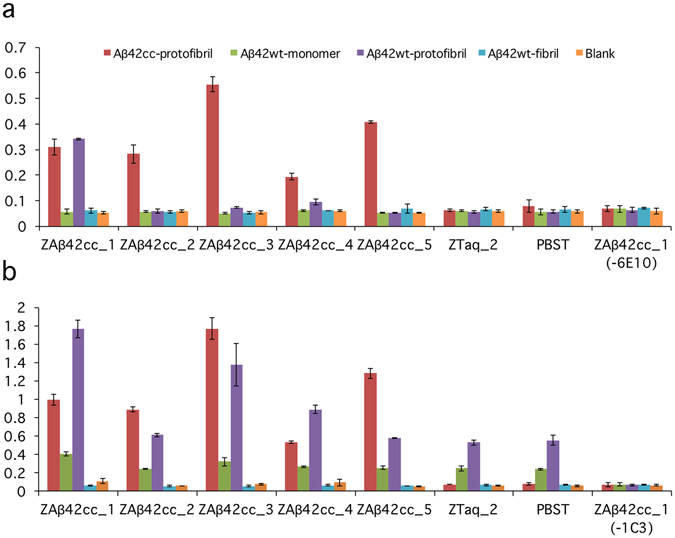
Binding profiling of five Affibody molecules to different Aβ aggregates, analyzed by ELISA. (a) Aβ42wt and Aβ42cc aggregates (50 nM assay concentration), bound to Affibody molecules, were detected by 6E10-HRP. 6E1021 recognizes the N-terminus of Aβ. As expected, all five Affibody molecules show binding to Aβ42cc protofibrils. The Affibody molecule ZAβ42cc_1 also binds to Aβ42wt protofibrils. No binding could be observed to either wild type monomer or fibrils. (b) Same as in (a), except that the assay concentration of Aβ42 was 1 µM and mAb1C3 was used for detection (weakly specific for protofibrils21). The binding profile has the same pattern as in (a) for protofibrils (both wt and cc) and Aβ42wt fibrils, but with a higher background for Aβ42wt protofibrils. (a,b) Two controls involve replacing the Affibody molecule with either an irrelevant Affibody molecule (ZTaq_2) or by PBS-T. In a third control experiment PBS-T was added instead of Aβ-specific antibody (6E10 or mAb1C3). Values are means of duplicate experiments.
