Figure 4.
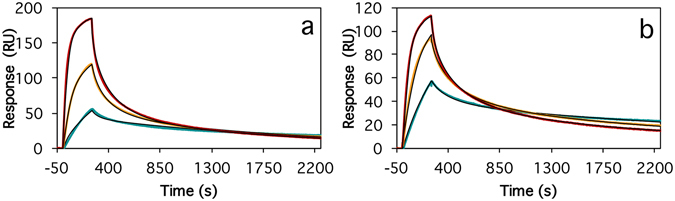
Association and dissociation kinetics for binding of two representative monomeric Affibody constructs to immobilized Aβ42cc protofibrils. Affibody concentrations are 10 (green), 20 (yellow) and 40 nM (red). The data was fitted to a heterogeneous ligand binding model with local maximum response (Rmax) values. The kinetics of the slow association and dissociation phases are k a = 2.8 (±0.1) × 105 s−1 M−1 and k a = 2.5 (±0.1) × 105 s−1 M−1 and k d = 4.7 (±0.05) × 10−4 s−1 and k d = 6.2 (±0.05) × 10−4 s−1 for ZAβ42cc_1 (a) and ZAβ42cc_4 (b), respectively, which correspond to dissociation constants for the stronger binding site of K D = 1.6 (±0.1) and K D = 2.5 (±0.2) nM, respectively.
