Figure 5.
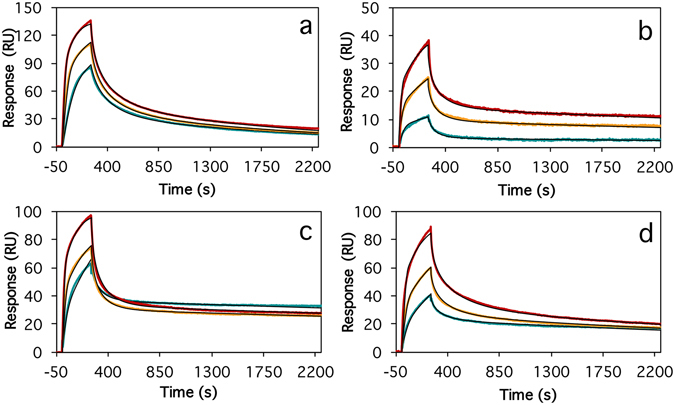
Association and dissociation kinetics for binding of some of the head-to-tail Affibody dimers to immobilized Aβ42cc protofibrils. Data were fit to a heterogeneous ligand binding model with local maximum response (Rmax) values. (a) ZAβ42cc_1-(GGGGS)- ZAβ42cc_1-ABD with Affibody dimer concentrations of 10 (green), 20 (yellow) and 40 nM (red). The kinetics of the slow association and dissociation phases are k a = 3.1 (±0.1) × 105 s−1 M−1 and k d = 5.2 (±0.03) × 10−4 s−1 corresponding to a dissociation constant for the stronger binding site of K D = 1.7 (±0.1) nM. (b) ZAβ42cc_1-(GGGGS)4- ZAβ42cc_1-ABD; 10, 20 and 40 nM; k a = 1.4 (±0.1) × 105 s−1 M−1, k d = 1.6 (±0.01) × 10−4 s−1, K D = 1.1 (±0.1) nM. (c) ZAβ42cc_4- ZAβ42cc_4 (no linker); 7.8, 15.5 and 31.2 nM; k a = 3.4 (±0.1) × 105 s−1 M−1, k d = 9.1 (±0.01) × 10−5 s−1, K D = 0.27 (±0.01) nM. (d) ZAβ42cc_4-(GGGS)4- ZAβ42cc_4; 7.8, 15.5 and 31.2 nM; k a = 2.2 (±0.3) × 105 s−1 M−1, k d = 2.5 (±0.01) × 10−4 s−1, K D = 1.1 (±0.05) nM.
