Figure 7.
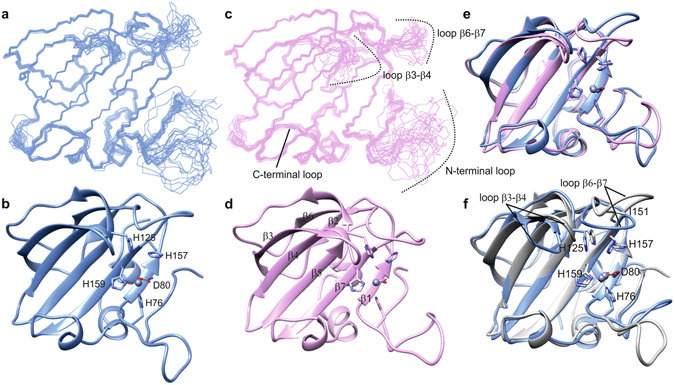
Structure of LytU and superposition to LytM catalytic domain. (a and c) Ensembles of fifteen structures of the least restraint violations of one- and two-zinc LytU, respectively. Backbone and heavy atom RMSDs for ordered residues of one- and two-zinc LytU are 0.3 and 0.7 Å, respectively. Residues shown are 57-192. (b and d) The lowest-energy structures represented in ribbon diagrams, for one- and two-zinc LytU, respectively. The substrate-binding groove can be seen horizontally, with the zinc-binding residues on the right. It is composed of four strands: β1:79-82, β3:95-103, β4:107-114, β5:119-129, and β7:158-164 and four loops: N-terminal loop: 64-78, loops β2-β3:104-106 and β6-β7:147-157, and C-terminal loop: 165-171, the latter of which includes a 310 helical stretch. (e) Superposition of one- (blue) and two-zinc (pink) LytU. (f) Superposition of 1Zn LytU (blue) and LytM (PDB ID 2B13, grey). LytU is strikingly similar to LytM with an RMSD over backbone atoms in secondary structures of 0.9 Å. Residues shown are the zinc-coordinating H76, D80, H159 as well as the catalytic H125 and H157. The zinc ion is shown as a grey sphere. The position of the dimorphic residue of LytU, I151 in this case, is indicated in the β6-β7 loop. The structure of two-zinc LytU was determined with restraints for the first, tighter binding zinc only, and therefore the second zinc is not present in panels 2d and 2e. The figure was generated with the program UCSF Chimera48.
