Figure 1.
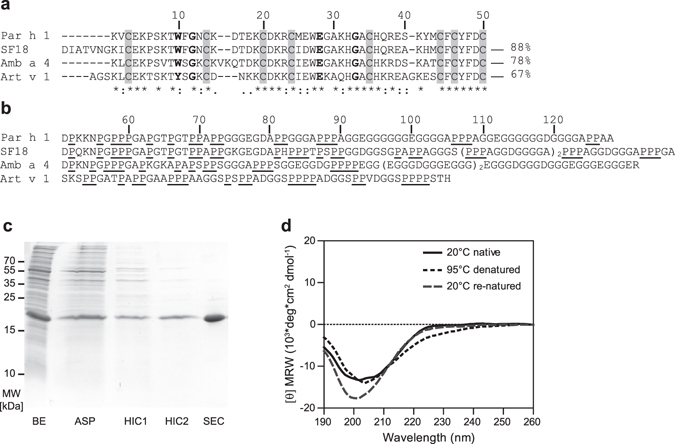
Sequence analysis, purification and secondary structure elements of recombinant Par h 1. (a) Sequence alignment of defensin-like domains obtained with the multiple sequence alignment program Clustal Omega (b), comparison of proline-rich regions. Art v 1 from Artemisia vulgaris (Q84ZX5), Amb a 4 from Ambrosia artemisiifolia (D4IHC0) and SF18 from Helianthus annuus (P22357) were included. Conserved cysteine residues boxed in grey, conserved residues of defensin-like proteins in bold, proline clusters underlined. The symbols shown in the alignment correspond with identical amino acids (*), conserved substitutions (:), and semi-conserved substitutions (.). (c) Purification process of recombinant Par h 1: BE, bacterial extract; ASP, ammonium sulfate precipitation; HIC1 and HIC2, hydrophobic interaction chromatography and SEC, size exclusion chromatography. (d) Circular dichroism spectra of purified Par h 1. The unprocessed gel is shown in the Related Manuscript File.
