Figure 1.
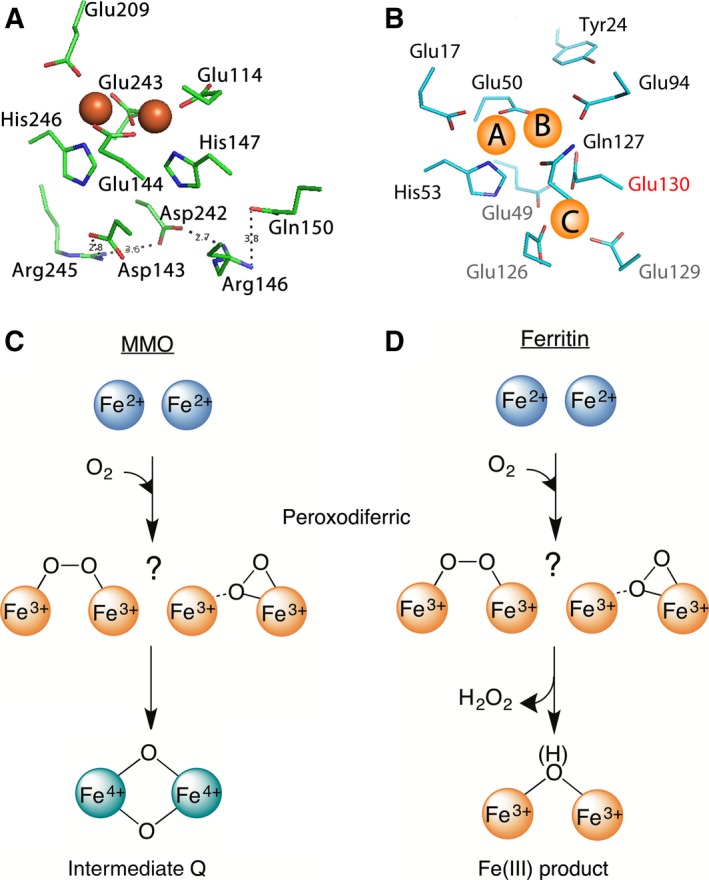
The di‐iron cofactor site of dioxygen‐activating enzymes is different from the di‐iron substrate site of ferritin. (A) The coordinating residues of the di‐iron cofactor site of MMO (PDB 1FYZ) are compared with (B) those of the di‐iron substrate site (the ferroxidase center) of PfFtn (PDB 2JD7). Three main differences are observed (see text). (C) In the di‐iron cofactor site of MMO the peroxodiferric intermediate (two possible molecular structures are shown) decays to intermediate Q, an antiferromagnetically coupled di‐iron Fe(IV) species with a bis‐μ‐oxo diamond core. (D) In ferritin, however, it is believed that the peroxodiferric intermediate directly decays to Fe(III) products.
