Figure 2.
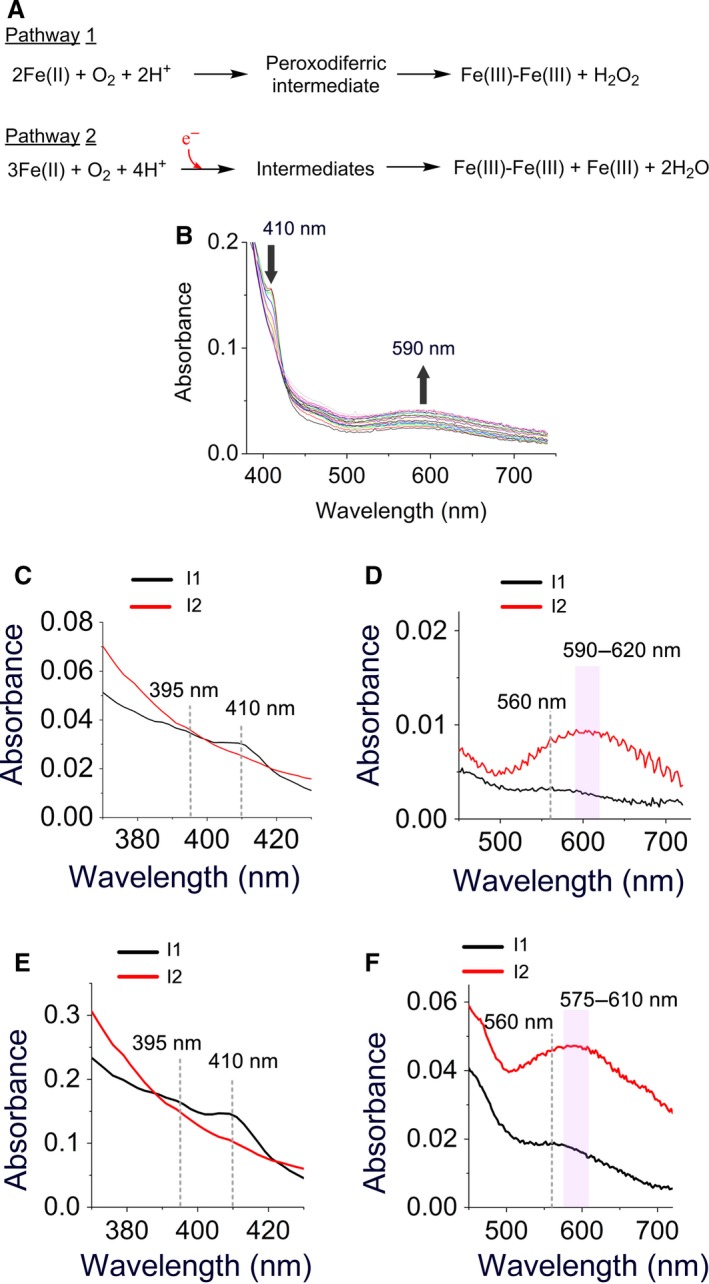
UV‐visible stopped‐flow spectroscopy suggests the presence of a new intermediate. (A) Two possible pathways proposed for the oxidation of Fe(II) in the ferroxidase center. Pathway 1 occurs in subunits whose sites A and B only are occupied with Fe(II) (AIIBIIC0 subunits) and pathway 2 occurs in subunits whose sites A, B, and C are occupied with Fe(II) (AIIBIICII subunits). In the second pathway the highly conserved tyrosine in the ferroxidase center is proposed to provide the fourth electron for a complete reduction of molecular oxygen to water. (B) UV‐visible absorbance spectra of different intermediates recorded for 5 s after the addition of circa 96 Fe(II) per ferritin 24‐mer to PfFtn. Measurements were performed at 47 °C. Concentration of PfFtn was 4.5 μm, which is 10 times less than the concentration of protein used for freeze‐quench experiments. The absorbance at 410 nm disappears after 5 s while the broad absorbance between 500 and 750 increases continuously. (C–F) The UV‐visible absorbance spectra of the intermediates during catalysis of Fe(II) oxidation by PfFtn were obtained using SVD analysis. (C–D) The absorbance spectrum of the intermediates obtained for the addition of 48 Fe(II) per ferritin 24‐mer (4.4 μm), or (E–F) for the addition of 96 Fe(II) per ferritin 24‐mer (15 μm).
