Figure 4.
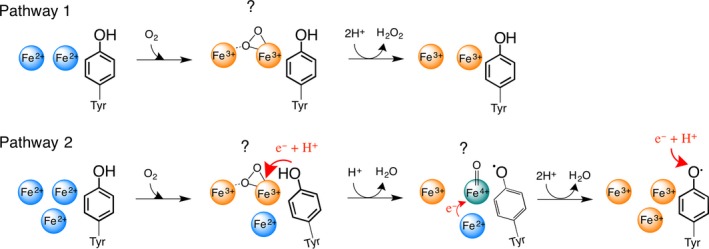
The proposed model for the role of the high‐valent Fe(IV) intermediate in the catalysis of Fe(II) oxidation. In pathway 1, when there is no extra Fe(II) nearby the ferroxidase center (site C), the two Fe(II) ions in the ferroxidase center are oxidized via a peroxodiferric intermediate to form the product. The bonding mode of dioxygen in peroxodiferric intermediate is under debate. Recent data based on X‐ray crystallography and Mössbauer spectroscopy suggest a μ‐η1‐η2 bonding mode. In pathway two, when extra Fe(II) is present in the vicinity of the ferroxidase center, first the two Fe(II) ions in the ferroxidase center form the peroxodiferric species. A conformational change occurs due to the presence of Fe(II) at site C. Consequently, the peroxodiferric species rapidly decays as the highly conserved tyrosine provides an electron and a proton. As a result a water molecule and an Fe(IV) intermediate species is formed. The exact binding mode of oxygen in Fe(IV) species is not known (?) and in the picture a terminal oxo group is shown for simplicity. The high‐valent Fe(IV) species then rapidly oxidizes the extra Fe(II) nearby to form a second water molecule and the Fe(III) products. Under reducing conditions the tyrosine radical is possibly reduced by an electron from a yet to be identified redox partner followed by the addition of a proton.
