Figure 1.
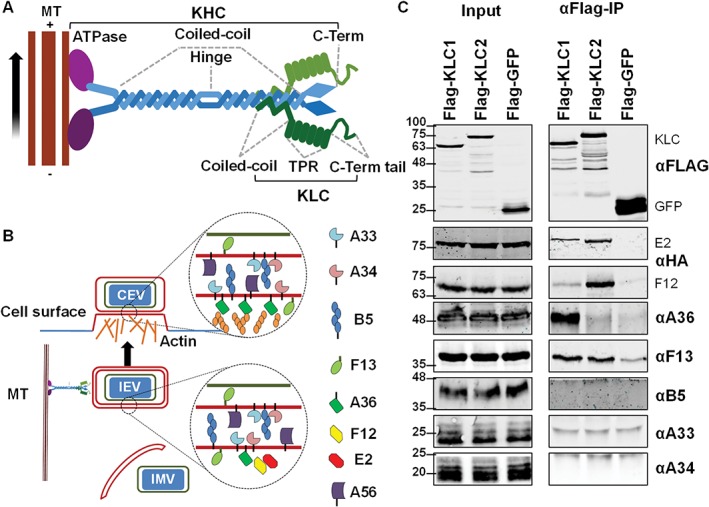
Testing the interaction of VACV IEV proteins with kinesin‐1 by co‐IP with epitope‐tagged KLC1 or KLC2. A, Schematic diagram of the kinesin‐1 complex that mediates trafficking of cargos along microtubules (MT) from the slow‐growing—end to the more dynamic + end oriented towards the cell periphery. Kinesin‐1 is usually represented as a heterotetramer consisting of 2 copies of KHC and 2 copies of KLC. KHC proteins (~110 to 130 kDa) possess an N‐terminal ATPase MT‐binding motor domain (shown in purple), a long coiled‐coil binding domain and C‐terminal tail domain of unknown structure. KLC proteins (51 to 76 kDa depending on the isoform) consist of a short N‐terminal coiled‐coil region responsible for binding to KHC, an α‐helix rich structural region consisting of 6 TPR motifs and a C‐terminal tail. B, The VACV IEV‐associated proteins. During infection some IMV are transported from virus factories on MTs and are wrapped by cellular membranes containing several VACV transmembrane and acylated proteins to form IEV particles. IEVs associate with the kinesin‐1 complex and are transported to the cell surface where F12/E2 dissociate. Virions are externalized by exocytosis and either remain bound to the cell surface as CEVs or are released as EEVs. CEVs can induce the polymerization of actin beneath the CEV particle and this requires the A36 protein. C, Co‐precipitation of IEV proteins with epitope‐tagged KLC1 or KLC2. FLAG‐tagged KLC1, KLC2 and GFP were expressed in HEK293T cells by plasmid transfection. Cells were infected 24 h later with VACV at 5 pfu/cell. Clarified cell lysates were generated 12 h post‐infection (hpi) and used to immunoprecipitate the FLAG‐tagged proteins. The immunoprecipitates were analysed by SDS‐PAGE and immunoblotting. Blots shown are representative of several experiments (n = 3) using either vF12‐HA or vE2‐HA.
