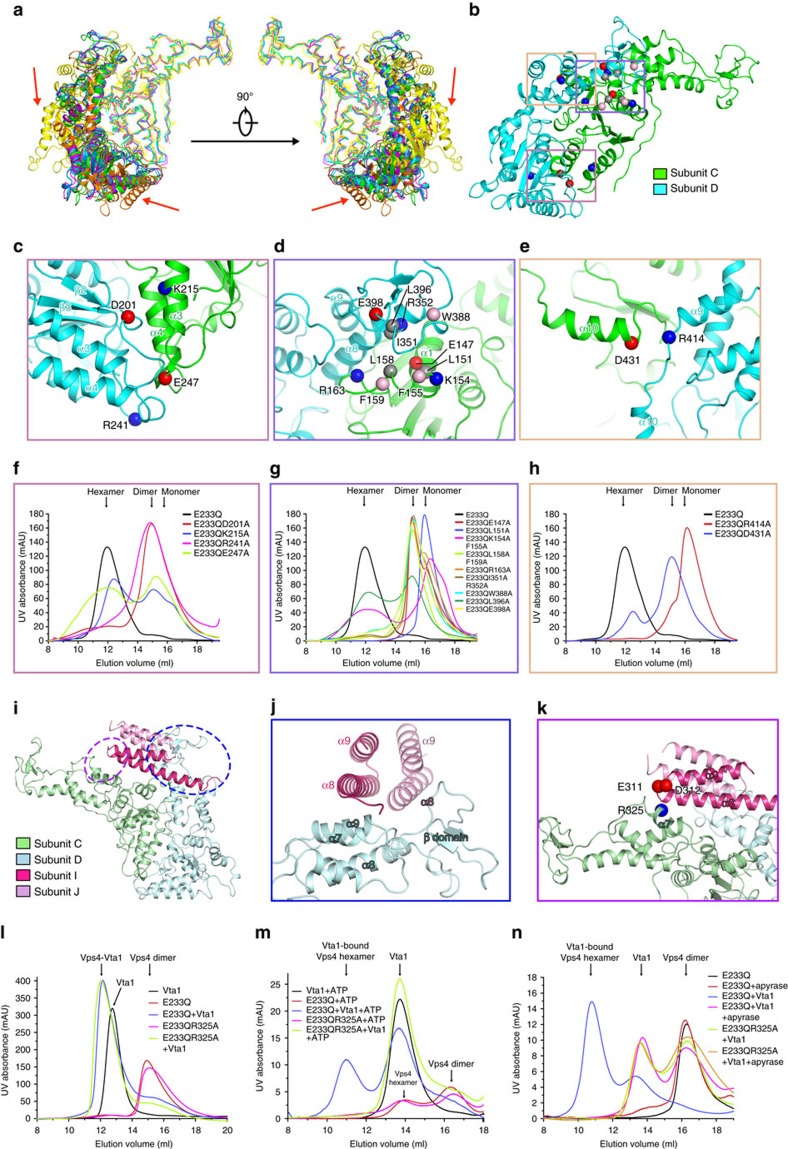
Figure 2. Characterizations of protein interactions in the Vta1-free and the Vta1-bound Vps4E233Q hexamers.
(a) Comparison of six pairs of the adjacent Vps4E233Q subunits. The colouring scheme is the same as in Fig. 1c,e. When one Vps4E233Q molecule (shown in ribbon representation) from each pair of the adjacent subunits aligned with each other, the other one (shown in cartoon representation) is also well aligned, except for the pairs involving subunit A (red arrow). (b) A focused view of the domains that mediate the interactions between the Vps4E233Q monomers (subunits C and D). Three boxed intermonomer interfaces are detailed in c–e. (c–e) The close-up views of the interfaces between the large ATPase domains (c), the large and small ATPase domains including the β-domain (d) and the C-terminal helixes (e). Potential residues participated in the interactions are represented by spheres. Hydrophobic, aromatic, positively charged and negatively charged residues are coloured grey, pink, blue and red, respectively. The orientations of all close-up views are optimized to show the interactions. (f–h) SEC analyses of the oligomeric states of the Vps4E233Q mutants targeting the interface between the large ATPase domains (f), the large and small ATPase domains including the β-domain (g) and the C-terminal helixes (h). (i) A focused view of the interactions between the Vta1 CTD dimer (subunits I and J) and the adjacent Vps4E233Q subunit pair (subunits C and D). Two different contact modes are highlighted by purple and blue dashed circles, respectively, and detailed in j,k. (j,k) The close-up views of the interactions between the Vps4E233Q subunit D and the CTD dimer (j), and the Vps4E233Q subunit C and the CTD dimer (k). (l,m) Assessments of the interactions by SEC between Vta1 and Vps4 mutants, E233Q and E233QR325A, at high concentration (100 μM) in the absence of ATP (l), and at low concentration (5 μM) in the presence of ATP (m). (n) Assessment of the stabilities of the Vta1-free Vps4E233Q hexamer and the Vta1-bound Vps4E233Q hexamer formed by different Vps4 mutants with or without apyrase treatment by SEC (See Methods for details).