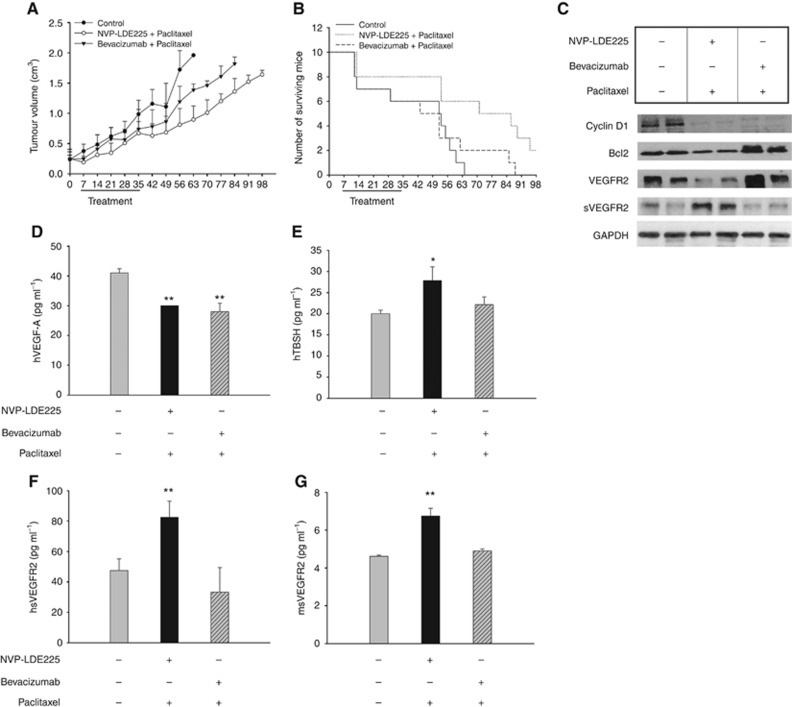
Figure 5.
NVP-LDE225 increases the efficacy of paclitaxel in nude mice xenografted with TNBC tumours. (A) Tumour volume of MDA-MB-468 orthotopic xenografts in nude mice, randomised (10 per group) to receive NVP-LDE225 or bevacizumab in combination with paclitaxel, as described in the Methods section. The one-way ANOVA test was used to compare tumour sizes among treatment groups at the median survival time of the control group (35 days). Comparison of tumour sizes, evaluated by the one-way ANOVA test, was statistically significant for the combination NVP-LDE225 and paclitaxel vs control (P⩽0.05). (B) Number of surviving mice orthotopically xenografted with MDA-MB-468, after treatments with NVP-LDE225 or bevacizumab in combination with paclitaxel, as described in the Methods section. Median survival differences were statistically significant median survival in the NVP-LDE225 plus paclitaxel-treated mice was significantly longer than in control mice (79.50 vs 53.50 days, P=0.0089, log-rank test). (C) Western blot analysis on total lysates from MDA-MB-468 tumour specimens of mice killed on day 21, after 2 weeks of treatment with NVP-LDE225 or bevacizumab in combination with paclitaxel. (D, E, F, G) ELISA assay for the determination of (D) hVEGF-A, (E) hTHSB1, (F) h-sVEGFR2 and (G) m-sVEGFR2 concentrations (pg ml−1) in mice sera, collected on day 21. Data represent the mean (±s.d.) of three independent experiments, each performed in triplicate. Bars, s.d. Asterisks indicate statistical significance, as determined by the Student t-test (*P<0.05, **P<0.01).