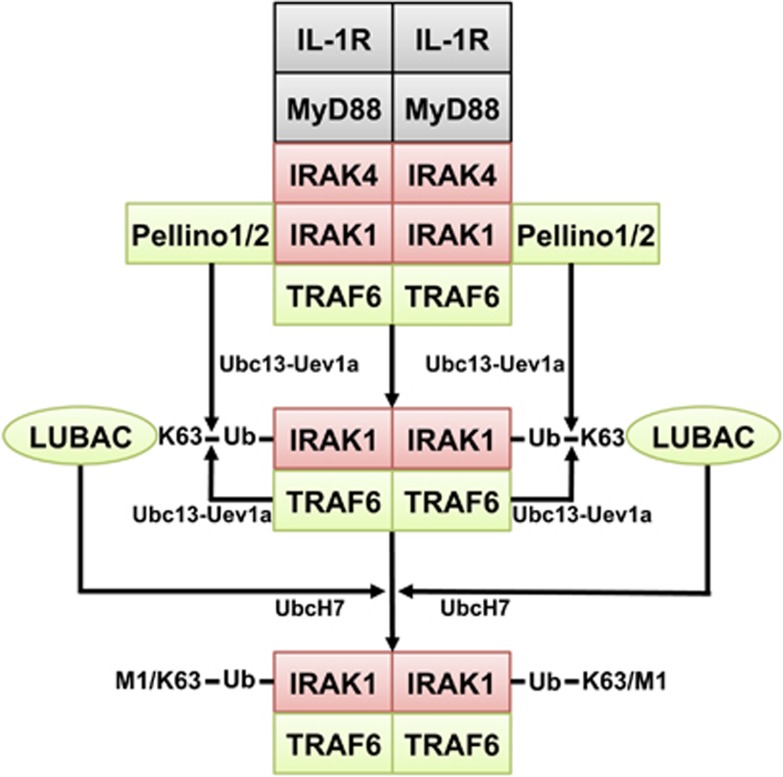
Figure 1.
How the MyD88 pathway triggers the ubiquitylation of IRAK1. The interaction of IL-1 with its receptor leads to the formation of the Myddosome and the phosphorylation and activation of IRAK1. The interaction of IRAK1 with TRAF6 induces dimerisation of the RING domain of TRAF6, activating its E3 ligase function. IRAK1 also phosphorylates Pellino1 and Pellino2 converting them from inactive to active E3 ubiquitin ligases. In the presence of E1-activating enzyme and the E2-conjugating complex Ubc13-Uev1a, TRAF6 and Pellinos 1 and 2 both contribute to the formation of K63-Ub chains, which become attached to IRAK1 and other components of the Myddosome. One essential role of TRAF6 is to recruit LUBAC into the IL-1 signalling complex where it interacts with K63-Ub-substrates, such as IRAK1, to produce K63/M1-Ub-IRAK1. In the human cell lines we have studied, the expression of IRAK1 is essential for IL-1 signalling and the IRAK2 in these cells cannot compensate for the loss of IRAK1. Protein kinases are highlighted in red and E3 ligases in green