Figure 3.
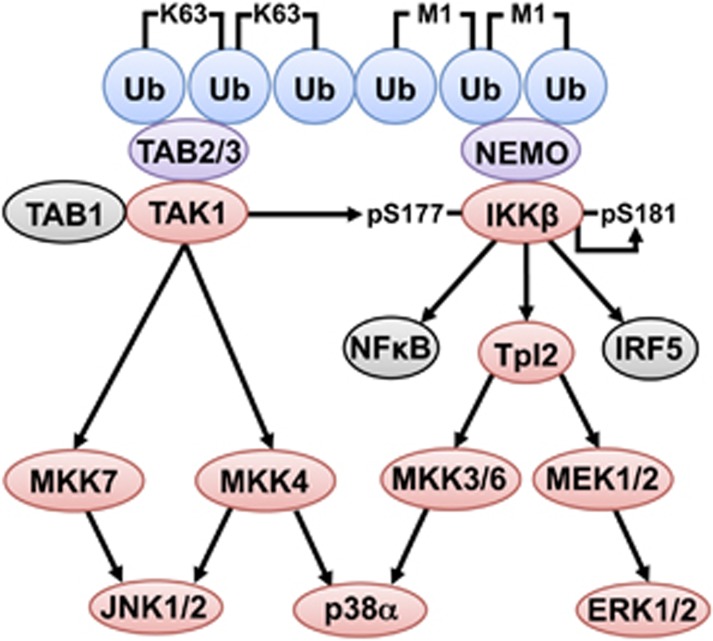
The formation of K63/M1-Ub hybrids facilitates the activation of TAK1 and IKK complexes, and the phosphorylation of their substrates. The K63/M1-Ub hybrids produced by innate immune signalling pathways recruit the TAK1 and IKK complexes. The interaction of K63-Ub chains with TAB2 and TAB3 activates TAK1 allowing it to phosphorylate and activate MAP kinase kinases 4 and 7 (MKK4, MKK7), which activate the MAP kinases, termed JNK1 and JNK2. The binding of M1-Ub chains to NEMO induces a conformational change that permits TAK1 to phosphorylate IKKβ at Ser177. IKKβ completes the activation process by phosphorylating itself at Ser181. IKKβ can then activate transcription factors essential for inflammatory mediator production (NF-κB and IRF5). IKKβ also activates the Tpl2 kinase complex, which activates MEK1 and MEK2, the protein kinases that activate ERK1 and ERK2. Tpl2 additionally phosphorylates MKK3 and MKK6. MKK3 and MKK6 operate redundantly with MKK4 to phosphorylate and activate p38α in some cells. Protein kinases are highlighted in red, ubiquitin-binding proteins in purple and ubiquitin (Ub) molecules in blue. Further details are given in the text