Figure 1.
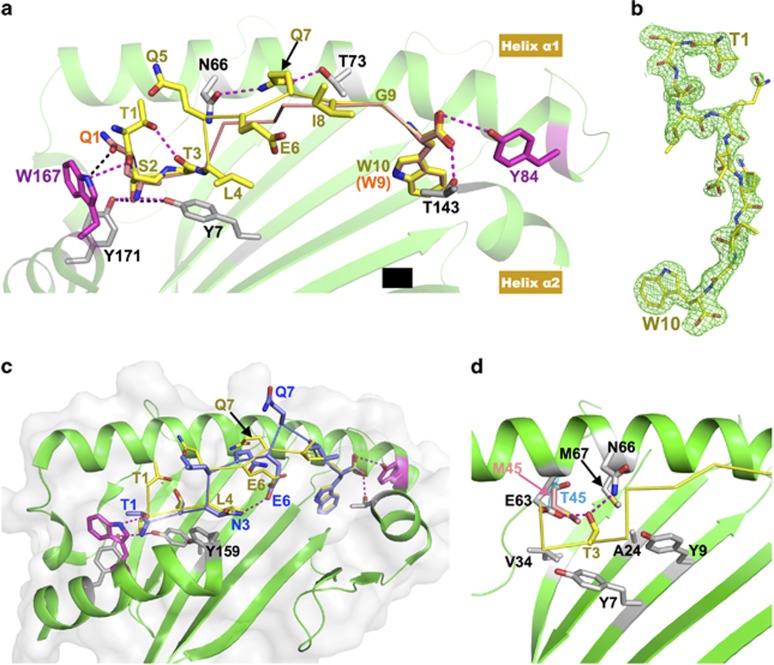
Structural features of immunodominant peptides bound to HLA-B*5801 and/or HLA-B*5701. (a) Comparison of two immunodominant antigenic peptides, TW10 and QW9, bound to the same HLA-B*5801 molecule. The green cartoon is the HLA-B*5801 structure. For clarity, the portion between Thr143 and Tyr171 of helix α2 was removed. The yellow stick/ribbon model is the TW10 peptide, and the orange stick/ribbon model is QW9. The two HLA residues that impose antigenic peptide length restriction, Trp167 and Tyr84, are highlighted with magenta sticks. The hydrogen bonds formed between TW10 and HLA-B*5801 are shown with magenta dashed lines, while the hydrogen bonds formed between Gln1 of QW9 and HLA-B*5801 are shown with black dashed lines. In TW10, the Ser2 side-chain forms two hydrogen bonds (magenta dashed lines) with Tyr7 and Tyr171 of HLA in place of the two canonical hydrogen bonds (black dashed lines) formed between the amino group of Gln1 of QW9 with Tyr7 and Tyr171. (b) 2Fo-Fc electron density map for TW10 bound to HLA-B*5801, contoured at 1.2 σ. The peptide TW10 (yellow stick) is depicted with its N-terminal Thr1 to the top right and its C-terminal Trp10 to the bottom. Notably, the electron density convincingly shows that the N-terminal residue Thr1 is not embedded within but instead bends away from the peptide-binding groove of the HLA molecule. (c) Overlay of the structure of HLA-B*5701/TW10-T3N onto that of HLA-B*5801/TW10-wt. HLA-B*5801 shown as a green ribbon drawing. For clarity, the nearly identical HLA-B*5701 molecule is not shown here. The yellow stick/ribbon model is TW10-wt, and the blue stick/ribbon model is TW10-T3N. The N-terminal amino group of TW10-T3N forms two canonical hydrogen bonds with the HLA molecule. Asn3 of TW10-T3N forms an internal hydrogen bond with Glu6 within TW10-T3N. Note that Gln7 of TW10-T3N prominently protrudes from the peptide-binding groove. (d) The environment of the peptide-binding pocket for the Thr3 residue of TW10. For clarity, the HLA-B*5701 molecule is not shown here. Thr3 forms hydrogen bonds with Glu63 and Asn66 located in helix α1 of HLA-B*57/5801. The peptide-binding pocket, to which the Thr3 side-chain points, is enriched in relatively hydrophobic residues. The Thr45 residue of HLA-B*5801 is shown in cyan, and Met45 of HLA-B*5701 at the same site is highlighted with a pink stick.