Autoimmune diseases have a strikingly higher prevalence in women compared to men, with approximately 80% of individuals affected with autoimmune diseases being female. The nature of this sex-bias has remained unclear, but sex hormones have previously been implicated as the leading underlying cause. In this research highlight, we discuss a recently identified transcription factor, vestigial like family member 3 (VGLL3), as a non-hormonally influenced regulator of the heightened inflammatory responses observed in women, and discuss its role as a potential driver of autoimmunity.
Sexual dimorphism, in which different characteristics are exhibited by the two sexes of the same species, has long fascinated scientists. One remarkable example is the anglerfish. While females measure approximately 10 cm in length, in some suborders the males can be 10 times smaller, with the record being 6.2 mm. The males are so tiny, living attached to females, that scientists wondered why all the anglerfish they captured were females until they noticed the small ‘growth’ on the female skin.
From the perspective of evolution, sexual dimorphisms are often explained by sexual selection, the selection arising from differential mating success. In the example of the anglerfish, it is thought that in the sparsely populated ocean, the chances for a male to find his mate are so low that once this happens, he attaches to her and never leaves. In this way he can provide her with sperm his entire life, and in return he gets all his nutrients from her. Without the need to hunt for food, he degenerates all his internal organs except the testes.
Sex differences in humans, although arguably subtler than those in anglerfish, are important in medicine. Drugs can require sex-specific dosing, and their effects can differ between men and women.1 Many diseases are more prevalent in one sex, with autoimmune diseases being one of the most striking examples. Systemic lupus erythematosus (SLE) has a female-to-male ratio of 9:1. The female-to-male ratio of scleroderma and Sjögren’s syndrome can be as high as 20:1, and that of Grave’s disease can reach 7:1.2 Overall, it is estimated that 78% of people affected with autoimmune diseases are women.2 By contrast, infectious diseases are more prevalent in men.3
Sexual selection has similarly operated during human evolution, as supported by sex differences in attributes such as physical size that are related to advantages in male−male competition and female choice.4 It has been proposed that the primary androgenic hormone, testosterone, can stimulate development of characteristics used in sexual selection while increasing risks of infection owing to its immunosuppressive effects.5 Indeed, testosterone has been shown to reduce natural killer-cell activity; expression of Toll-like receptors (TLR); and synthesis of tumor necrosis factor (TNF), inducible nitric oxide synthase and nitrogen oxide by macrophages.6, 7, 8 Autoimmune regulator (AIRE), a key factor in central tolerance that promotes self-antigen expression in medullary thymic epithelial cells, has been shown to be upregulated by androgens and downregulated by estrogen.9, 10 However, intriguingly, in humans, mutations in AIRE, which cause autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, are characterized by combinations of autoimmune diseases such as Addison’s disease, vitiligo, type I diabetes and alopecia, which are diseases that do not generally show gender bias.11
To understand the mechanism of sex differences in susceptibility to immune diseases, we have investigated sexual dimorphisms in immune processes on a molecular level.12 Using high-resolution transcriptome analyses, we found 661 genes that are expressed differentially by the two sexes in human skin. The female-biased genes (that is, genes expressed more highly in females than in males) were significantly enriched for processes known to be dysregulated in autoimmune diseases, including complement activation and phagocytosis regulation. By contrast, the male-biased genes were enriched for transcription regulation and development. Importantly, the female-biased genes were significantly associated with common disease loci of SLE and systemic sclerosis, two female-dominant autoimmune diseases. The female-increased genes included BAFF/TNFSF13B, the target for the first biologic therapy approved for SLE, and ITGAM, whose variants are associated with SLE susceptibility from genome-wide association studies.13, 14
Further analysis of the data demonstrated a genome-wide co-expression network that extended beyond the 661 sex-biased genes. This network involved multiple inflammatory pathways, indicating the presence of extensive genome-wide differences in immune regulation between the two sexes.
Interestingly, we demonstrated that sex hormones do not affect the expression of the female-biased genes that are involved in immune processes. Consistently, the expression of these genes did not decrease with age, despite a decrease in estrogen levels after menopause. These findings raise the possibility that sex-bias in immune regulation is independent of sex hormones and is further corroborated by various clinical studies showing lack of association between the onset or activity of autoimmune diseases with sex hormone levels.15
By screening transcription factors that demonstrate significant female-bias in human skin, we identified VGLL3 as the only transcription factor that was required for the expression of a large set of female-biased immune genes, including BAFF/TNFSF13B, ITGAM and C3. Human VGLL3 was originally identified as a homolog to the Drosophila gene vg (‘vestigial’), which encodes a cofactor of Scalloped, a homolog of the transcriptional enhancer TEF-1.16 Notably, VGLL3 in salmon exhibits sex-dependent dominance, promoting earlier and later maturation in males and females, respectively.17 This suggests that the role of VGLL3 in regulating sexual dimorphism is evolutionarily conserved.
In primary human keratinocytes, VGLL3 knockdown reduced the expression of 7 out of 10 keratinocyte-expressed genes that are associated with female-biased diseases. On a genome-wide level, all of the top ten biological pathways enriched in VGLL3 targets are related to immune processes. In addition, ‘autoimmune diseases’ was among the top disease categories enriched, involving 47% of VGLL3-regulated genes.
In subacute cutaneous lupus erythematosus (SCLE), a female-biased, lupus-specific skin eruption, the disease-upregulated genes showed a strong correlation with VGLL3-regulated genes. Furthermore, VGLL3 knockdown in SCLE keratinocytes significantly reversed the abnormality in gene expression. Notably, active disease was associated with increased VGLL3 protein expression and nuclear localization, consistent with increased activation of this pathway in inflamed tissue. Similarly, transcriptional upregulation by VGLL3 was associated with active states of morphea and systemic sclerosis, two female-biased autoimmune diseases of the skin.18 Importantly, VGLL3 was mostly cytoplasmic (transcriptionally inactive) in healthy male skin, but in males with SCLE, it was noted to be nuclear and likely participatory in disease.
Is the role of VGLL3 in autoimmune regulation restricted to the skin? In Sjögren’s syndrome, an autoimmune condition of the salivary and lacrimal glands that disproportionally affects more women than men at a 20:1 ratio,19 the expression of VGLL3 and its targets was increased. Many sexually dimorphic autoimmune diseases have in common a prominent activation of both type I and type II interferon (IFN) signaling. Notably, VGLL3 disruption by RNAi impaired the response of cultured salivary gland cells to IFN-α and IFN-γ. The regulation of interferon responses by VGLL3 is conserved in monocytes as well, as VGLL3 knockdown reduced the induction of bona fide type I interferon response genes. This suggests that one mechanism by which VGLL3 promotes development of autoimmune diseases is through heightened interferon responses.
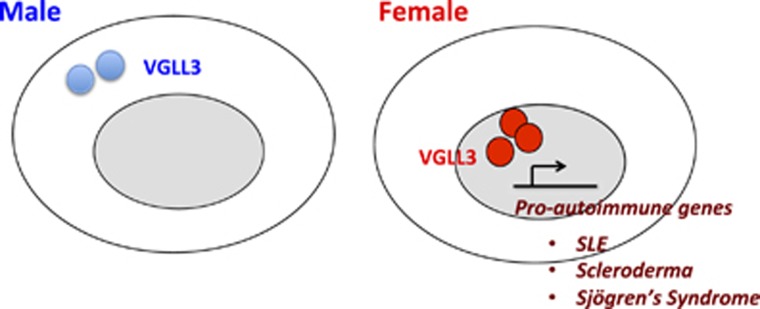
Since the discovery of sex differences in susceptibility to immune-associated diseases, many studies have suggested the differential regulation of immune genes such as TLRs, CCLs, CXCLs and ILs, in the two sexes.3 These studies have further suggested that hormonal and genetic mediators on the sex chromosomes modulate these differences.3 We have extended these findings and established a significant association between sex-biased immune gene expression in the human skin and disease loci for autoimmunity. In addition, we have demonstrated that the expression of these genes, the majority of which are located on autosomes, are not affected by sex hormones and biological age. The identification of VGLL3 as their upstream regulator provides a new, sex hormone-independent perspective to the molecular basis of sex disparities in immune gene regulation (Figure 1).
Figure 1.
VGLL3-regulated gene network promotes autoimmunity. The female-biased expression and nuclear localization of VGLL3 leads to elevated expression of pro-autoimmune genes, which may help explain the increased susceptibility of females to autoimmune diseases.
Decades ago, it was proposed that men were more susceptible to infection because their larger body size provided more ‘bites’ to bacteria. Over the years, we have made substantial progress in understanding the mechanisms underlying sex biases in immune responses, but we still have many more questions ahead of us to answer. For example, what is the mechanism of gene regulation by VGLL3? How is VGLL3 itself regulated in the two sexes? What is the role of VGLL3 in innate and adaptive immune responses? By understanding sex as a biological variable in immune responses and the pathogenesis of immune diseases, we may one day find a way to successfully combat or even prevent these diseases.
Acknowledgments
The work is supported by the US National Institutes of Health (K08-AR060802 and R01-AR069071to JEG; and R03-AR066337 and K08-AR063668 to JMK), an A Alfred Taubman Medical Research Institute Kenneth and Frances Eisenberg Emerging Scholar Award (JEG), the Doris Duke Charitable Foundation (2013106 to JEG) and a Pfizer Aspire Award (JEG).
Footnotes
The authors declare no conflict of interest.
References
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014; 509: 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol 2001; 2: 777–780. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16: 626–638. [DOI] [PubMed] [Google Scholar]
- Geary DC. Sex differences in social behavior and cognition: utility of sexual selection for hypothesis generation. Horm Behav 2006; 49: 273–275. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat 1992; 139: 603–622. [Google Scholar]
- Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008; 78: 432–437. [DOI] [PubMed] [Google Scholar]
- Hou J, Zheng WF. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol 1988; 10: 15–22. [DOI] [PubMed] [Google Scholar]
- D'Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann NY Acad Sci 1999; 876: 426–429. [DOI] [PubMed] [Google Scholar]
- Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun 2016; 7: 11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest 2016; 126: 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen J, Peterson P. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun 2003; 4: 12–21. [DOI] [PubMed] [Google Scholar]
- Liang Y, Tsoi LC, Xing X, Beamer MA, Swindell WR, Sarkar MK et al. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat Immunol 2017; 18: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 2014; 10: 365–373. [DOI] [PubMed] [Google Scholar]
- International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN)International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN)Harley JB International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN)Alarcón-Riquelme ME International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) Criswell LA International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN)Jacob CO International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN)Kimberly RP et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 2008; 40: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CR, Edwards CJ. The effects of hormone replacement therapy on autoimmune disease: rheumatoid arthritis and systemic lupus erythematosus. Climacteric 2009; 12: 378–386. [DOI] [PubMed] [Google Scholar]
- Maeda T, Chapman DL, Stewart AF. Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J Biol Chem 2002; 277: 48889–48898. [DOI] [PubMed] [Google Scholar]
- Barson NJ, Aykanat T, Hindar K, Baranski M, Bolstad GH, Fiske P et al. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 2015; 528: 405-+. [DOI] [PubMed] [Google Scholar]
- Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol 2009; 145: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Shahane A. The epidemiology of Sjogren's syndrome. Clin Epidemiol 2014; 6: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]