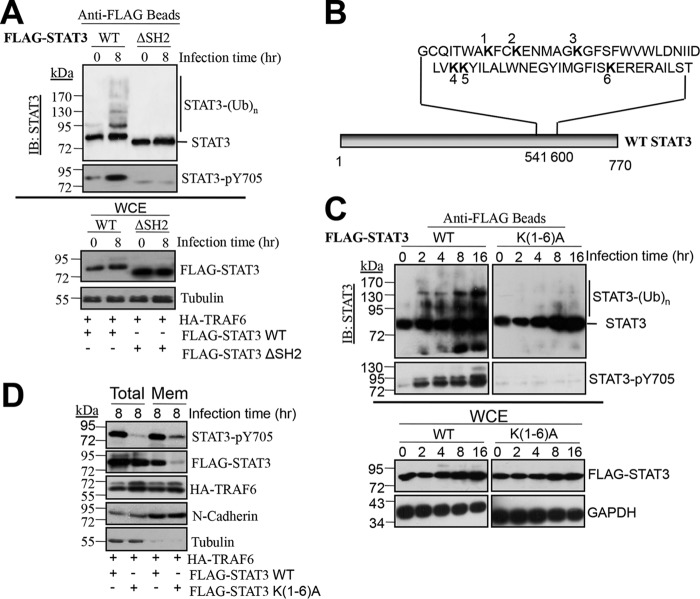
FIG 5.
Mutation of lysines within the SH2 domain abolishes STAT3 ubiquitination. (A) Ubiquitination and phosphorylation of FLAG-STAT3 or FLAG-STAT3 ΔSH2 when cotransfected with HA-TRAF6 into HEK293T cells for 24 h before infection with S. Typhimurium for 8 h. FLAG-STAT3 or FLAG-STAT3 ΔSH2 was pulled down using anti-FLAG M2 magnetic beads and was then analyzed with rabbit anti-STAT3 and rabbit anti-STAT3-pY705. WCEs were probed with mouse anti-FLAG and rabbit anti-tubulin (as a loading control). (B) Diagram indicating the locations of lysine residues within the SH2 domain of STAT3. (C) Effects of mutations in the STAT3 SH2 domain lysine residues on STAT3 ubiquitination and phosphorylation. FLAG-STAT3 or FLAG-STAT3 K(1-6)A was cotransfected with HA-TRAF6 into HEK293T cells for 24 h before infection with S. Typhimurium for the indicated times. FLAG-STAT3 was pulled down and detected as described for panel A. (D) Effects of mutations in the STAT3 SH2 domain lysine residues on STAT3 membrane localization and phosphorylation. FLAG-STAT3 or FLAG-STAT3 K(1-6)A was cotransfected with HA-TRAF6 into HEK293T cells for 24 h before infection with S. Typhimurium for 8 h. WCE and plasma membrane (Mem) fractions were analyzed by Western blotting with the indicated antibodies.