FIG 6.
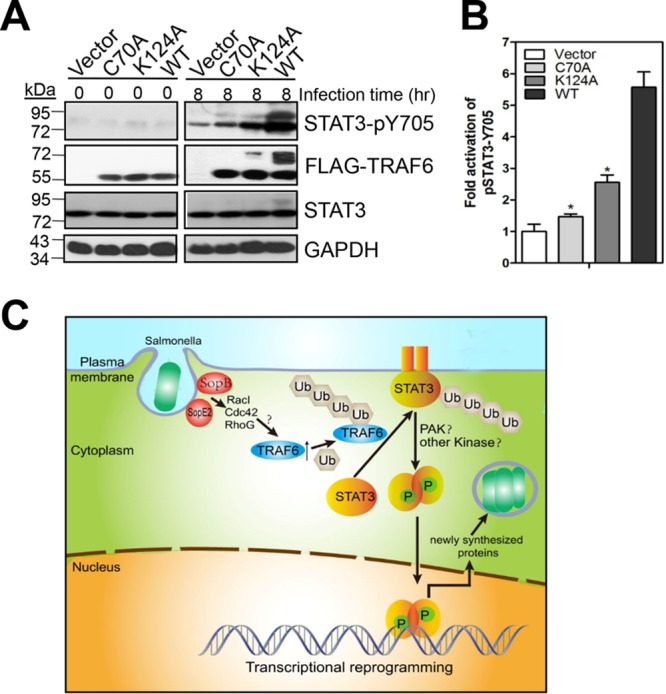
TRAF6 ubiquitination is important for the induction of STAT3 phosphorylation by S. Typhimurium. (A) Importance of the TRAF6 K124 residue in STAT3 phosphorylation. Traf6−/− MEFs were transfected with mock plasmids, pcDNA4-FLAG-TRAF6, pcDNA4-FLAG-TRAF6 C70A, or pcDNA4-FLAG-TRAF6 K124A for 24 h before infection with S. Typhimurium for 8 h. WCEs were probed with mouse anti-FLAG, rabbit anti-pSTAT3 (Y705), rabbit anti-STAT3, or anti-GAPDH (as a loading control). (B) Quantification of the fold activation of STAT3-pY705 (relative to that for Traf6−/− MEFs expressing a mock plasmid). Values are means (± SD) for three independent experiments. Asterisks indicate statistically significant (P < 0.05) differences from the values for Traf6−/− MEFs expressing wild-type TRAF6 as determined by Student's t test. (C) Schematic representation. SPI-1 T3SS effectors SopB and SopE2 initiate the ubiquitination of TRAF6 via the activation of RacI, Cdc42, and RhoG. TRAF6 catalyzes STAT3 ubiquitination, which is essential for STAT3 membrane recruitment and subsequent phosphorylation. TRAF6 ubiquitination not only acts as a hallmark of activation of ubiquitin ligase activity but also is involved in TRAF6-catalyzed STAT3 ubiquitination. STAT3 phosphorylation modulates host gene expression to promote intracellular bacterial replication.
