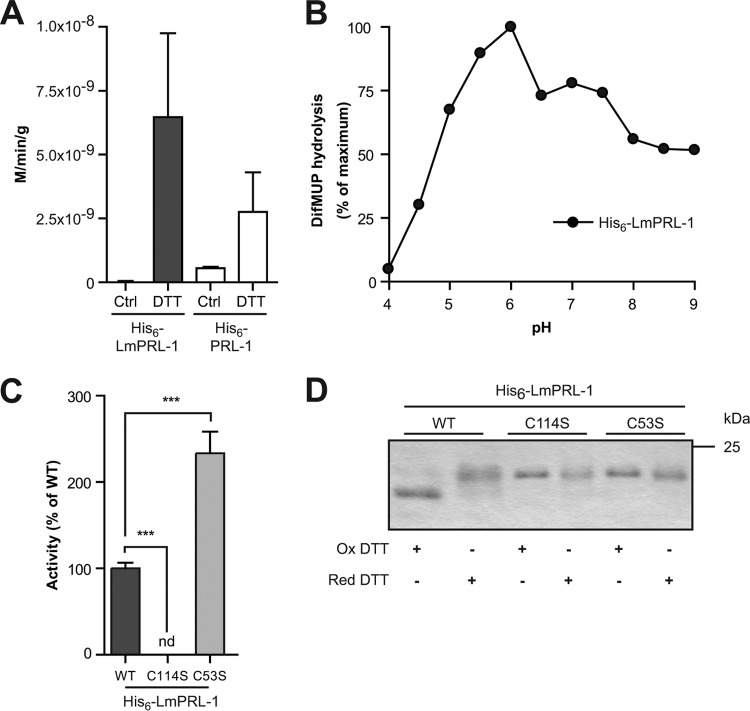
FIG 2.
Biochemical characterization of His6-tagged LmPRL-1. (A) Phosphatase activity of WT His6-LmPRL-1 against pNPP compared to the activity of the human His6-PRL-1. The phosphatase activity was measured by incubating His6-tagged LmPRL-1 (black bars) and PRL-1 (white bars) with pNPP for 1 h at 37°C and pH 6.0. Some reactions were carried out with previously reduced proteins. (B) Phosphatase activity of WT His6-LmPRL-1 against DiFMUP as a function of pH. His6-tagged LmPRL-1 was reduced before its phosphatase activity was measured by incubating it with DiFMUP for 20 min at 37°C and pHs varying from 4.0 to 9.0. (C) Effects of the catalytic (C114) and regulatory (C53) cysteines on the phosphatase activity of His6-LmPRL-1. The phosphatase activities of His6-LmPRL-1-C114S and His6-LmPRL-1-C53S (light-gray bar) against DiFMUP at pH 6.0 after 20 min at 37°C were measured and compared to the activity of the wild-type His6-LmPRL-1 (dark-gray bar). (D) Change of the electrophoretic migration of LmPRL-1 induced by oxidation is controlled by the catalytic and regulatory cysteines. The wild type and the catalytic and regulatory mutants of LmPRL-1 were either oxidized with 10 mM oxidized (Ox) DTT or reduced with 10 mM reducing (Red) DTT for 1 h at 4°C. The proteins were separated on nonreducing SDS-PAGE and dyed by Coomassie staining. The data represent the means and SD of the results of one of three experiments with similar results. ***, P < 0.001; nd, not determined.