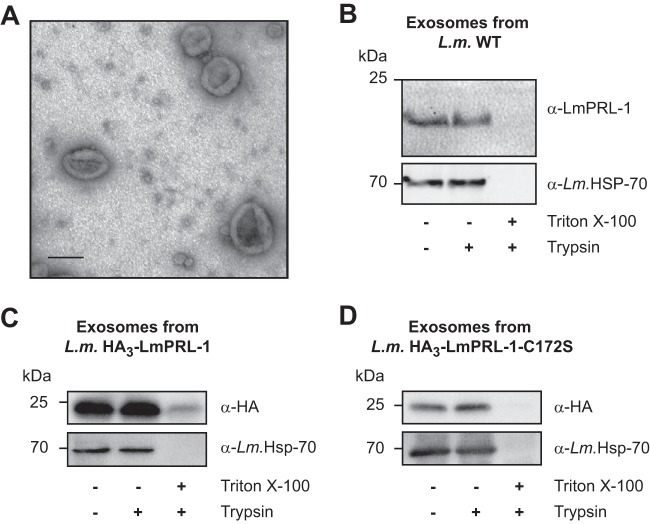
FIG 4.
LmPRL-1 is secreted by L. major promastigotes via exosomes. (A) Electron micrograph of exosomes produced by WT L. major promastigotes. Exosomes were purified from a culture of metacyclic promastigotes incubated overnight in serum-free RPMI 1640 medium at 28°C and then fixed and analyzed by electron microscopy. Bar, 100 nm. (B) Endogenous LmPRL-1 is secreted in exosomes of WT L. major (L.m.) promastigotes. Purified exosomes from WT promastigotes were analyzed by immunoblotting for the presence of LmPRL-1 and the exosome marker HSP-70. Untreated exosomes were compared to exosomes digested with trypsin or with trypsin in the presence of the detergent Triton X-100. (C) Ectopic expression and HA tagging does not alter LmPRL-1 secretion via exosomes. Purified exosomes from transfected L. major ectopically expressing HA3-LmPRL-1 were analyzed by immunoblotting for the presence of HA-tagged protein and the exosome marker HSP-70 following treatment similar to that described for panel B. (D) HA-tagged LmPRL-1 is secreted in exosomes independently of its farnesylation motif. Purified exosomes from transfected L. major ectopically expressing HA3-LmPRL-1-C172S were analyzed as described for panel C. The data presented are from one of three experiments with similar results.