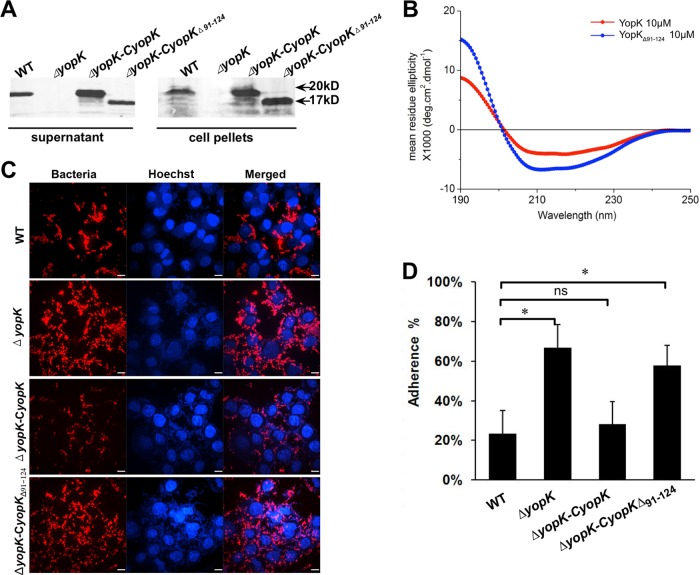
FIG 4.
Expression of YopKΔ91–124 in the ΔyopK strain does not restore a wild-type bacterial adhesion phenotype. (A) The Y. pestis strains were cultured in TMH medium without calcium at 37°C to an OD of 1.0. Bacterial cells and culture supernatants were collected by centrifugation, and the proteins were analyzed by SDS-PAGE and immunoblotting using anti-YopK antibody. YopKΔ91–124 was secreted by ΔyopK-CyopKΔ91–124 at a level comparable to that of YopK secretion by the wild-type Y. pestis. (B) CD profiles in the far-UV range (190 to 250 nm) of YopK and YopKΔ91–124 at 10 μM. The profiles represent the mean CD profiles for 8 measurements per protein. The buffer spectrum was subtracted from the proteins' spectra, and the resulting spectrum was analyzed with the online Dichroweb server. (C) HeLa cells were infected with the indicated strains at an MOI of 100 in the presence of 1 μM cytochalasin D. The percentage of adhesion was determined as described above. (D) HeLa cells were infected as described for panel C. After 2 h of infection, cells were extensively washed in prewarmed PBS to remove unattached bacteria, and the bacteria that adhered to HeLa cells were visualized using anti-F1 antigen monoclonal antibody and donkey anti-mouse IgG secondary antibody conjugated to Alexa Fluor 555. Scale bars in the images represent 9 μm. All experiments were independently performed at least three times in triplicate. Statistical analysis was performed using one-way ANOVA with Dunnett's multiple-comparison tests to analyze the significance of differences in bacterial adhesion between the different strains (*, P < 0.05; ns, not significant).