Figure 2.
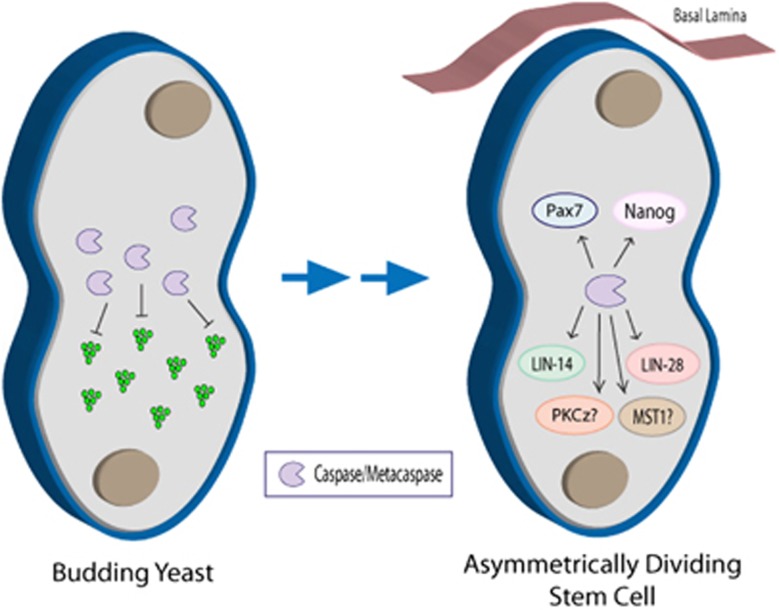
The role of caspase in establishing asymmetric cell division: potential primordial link between caspase function and cell differentiation. Caspase-dependent protein processing in the budding yeast, S. cerevisiae, is essential for proteostasis and the destruction of protein aggregates that would be detrimental to cell survival. Upon cytokenesis, the yeast metacaspase, Yca1, is tasked at preventing accumulated protein aggregates from entering the newly formed daughter cell, thereby increasing progeny fitness. This is similar to the emerging role of eukaryotic caspases in regulating stem cell symmetrical/asymmetrical division. To date, caspases have been associated with the degradation of the ESC pluripotency factor, Nanog, as well as the muscle cell lineage-specific factor, Pax7. Caspase targeting of these factors is essential for establishing asymmetric cell division and the initiation of cell differentiation. There is also evidence that suggests that caspase cleavage activation of PKCζ (PKCz in figure) – a known factor in mediating polarity cues in differentiating neuroblasts119 – is critical in initiating asymmetric cell division. Moreover, activation of the mammalian Ste20-like kinase (MST1) via caspase cleavage, which was recently established to be important to the fidelity of muscle cell differentiation, may be involved in polarity signaling during asymmetric cell division. This is based on previous work on Drosophila neuronal cells, where polarity cues relied, in part, on the activated homolog for MST1, Hippo.120, 121 The conservation of caspase involvement in cell polarity signaling is further supported by the caspase-dependent cleavage of LIN-14 and LIN-28 in C. elegans seam cells, with both proteins being critically involved in symmetric/asymmetric cell divisions. Caspase-dependent protein processing during asymmetric cell division may represent the origins of caspase involvement in cell differentiation, and may precede the death-centric functions of these proteases