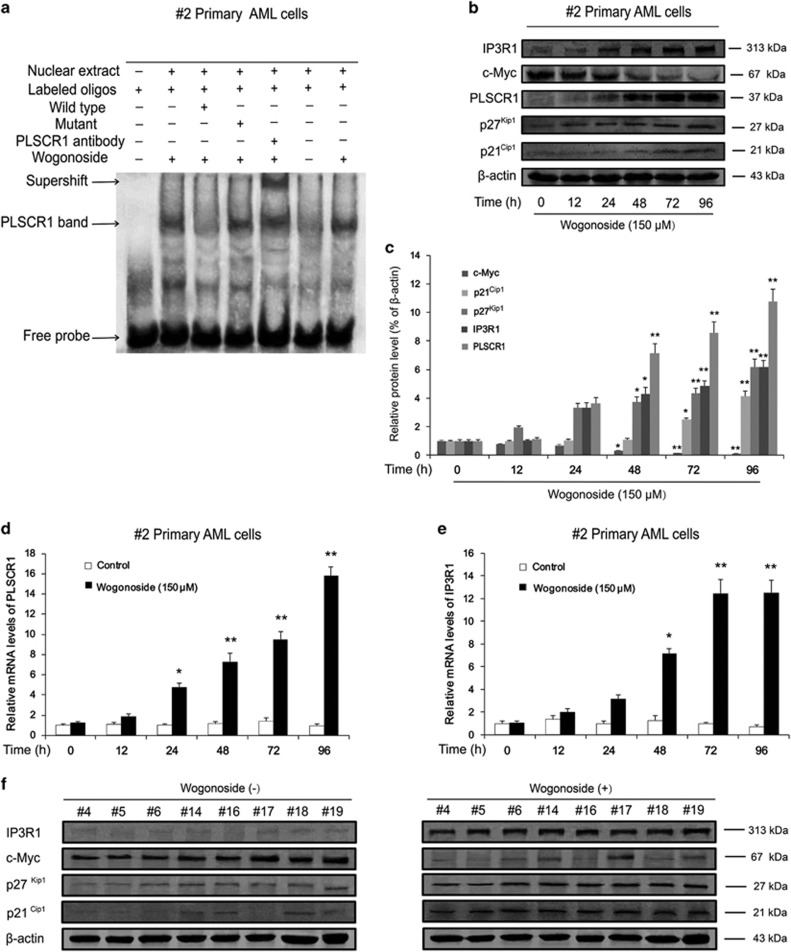
Figure 2.
Wogonoside facilitates PLSCR binding to the IP3R1 promoter and influences the expression of cell cycle- and differentiation-related proteins and genes in primary AML cells. (a) Data of EMSA assay to detect PLSCR1 binding to its consensus site in the IP3R1 promoter is shown. #2 Primary AML cells were incubated with wogonoside (150 μM) for 48 h, and DNA binding was determined in nuclear extracts using EMSA. To determine the composition of the DNA-binding complex, the anti-PLSCR1 antibody was used for supershift experiments. Data are representative of three separate experiments. (b and c) #2 Primary AML cells were treated with or without 150 μM wogonoside for 0, 12, 24, 48, 72 and 96 h. Whole-cell extracts at different time points were analyzed by western blot for PLSCR1 and cell cycle- and differentiation-related proteins, including p21Cip1, p27Kip1, c-Myc, IP3R1, using β-actin as a loading control. In western blot, the amounts of cell extract in each gel were exactly equal in analysis for purpose proteins; moreover, the experiment condition and scanning parameter were permanent. The data represent the mean±S.E.M. of three different experiments. Asterisks denote statistically significant (*P<0.05 and **P<0.01) differences compared with controls by one-way ANOVA. (d and e) Total RNAs were extracted at the indicated time points. PLSCR1 and IP3R1 mRNA levels were detected by quantitative real-time reverse transcription-PCR, and fold changes were assessed and shown normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA level. For analysis of RT-PCR results, asterisks denote significant (*P<0.05 and **P<0.01) differences relative to controls by two-tailed Student’s tests. (f) Primary AML samples (#4, #5, #6, #14, #16, #17, #18 and #19) were treated with or without 150 μM wogonoside for 96 h. Whole-cell extracts at different time points were analyzed by western blot for PLSCR1 and cell cycle- and differentiation-related proteins, including p21Cip1, p27Kip1, c-Myc and IP3R1, using β-actin as a loading control