Abstract
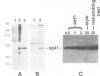
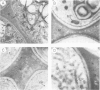
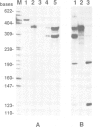
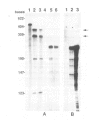
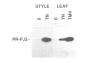
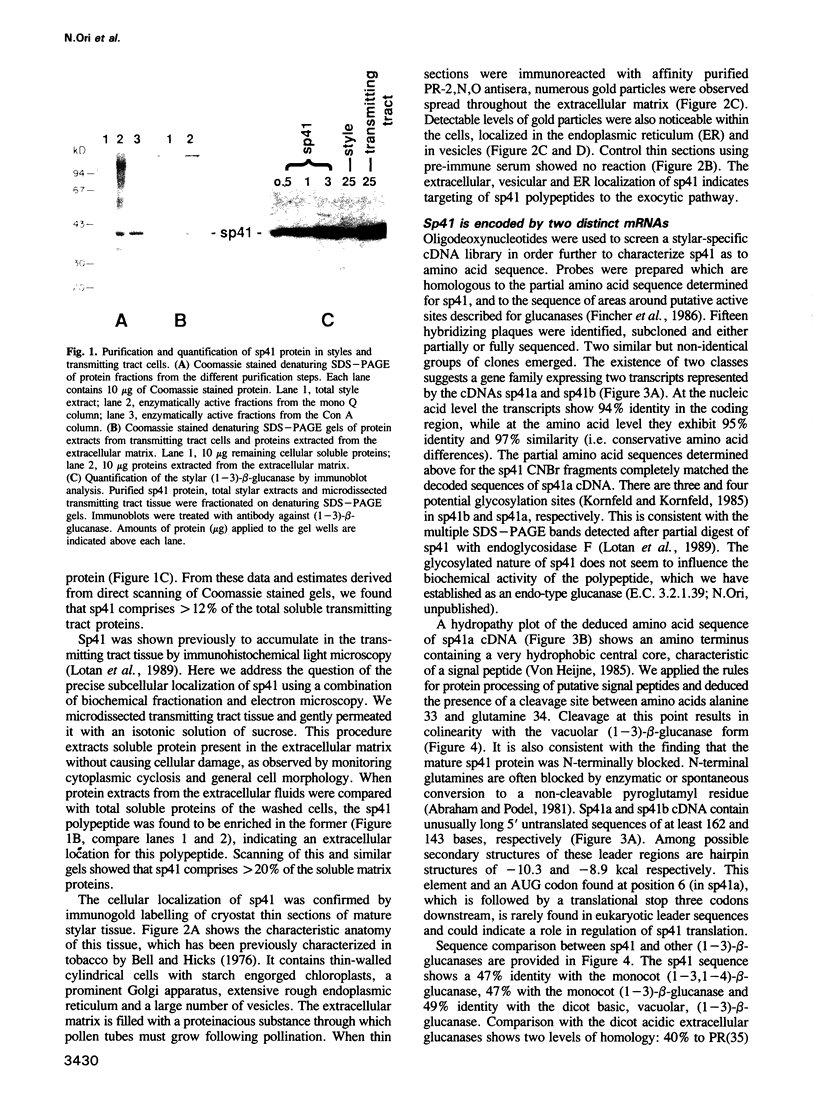
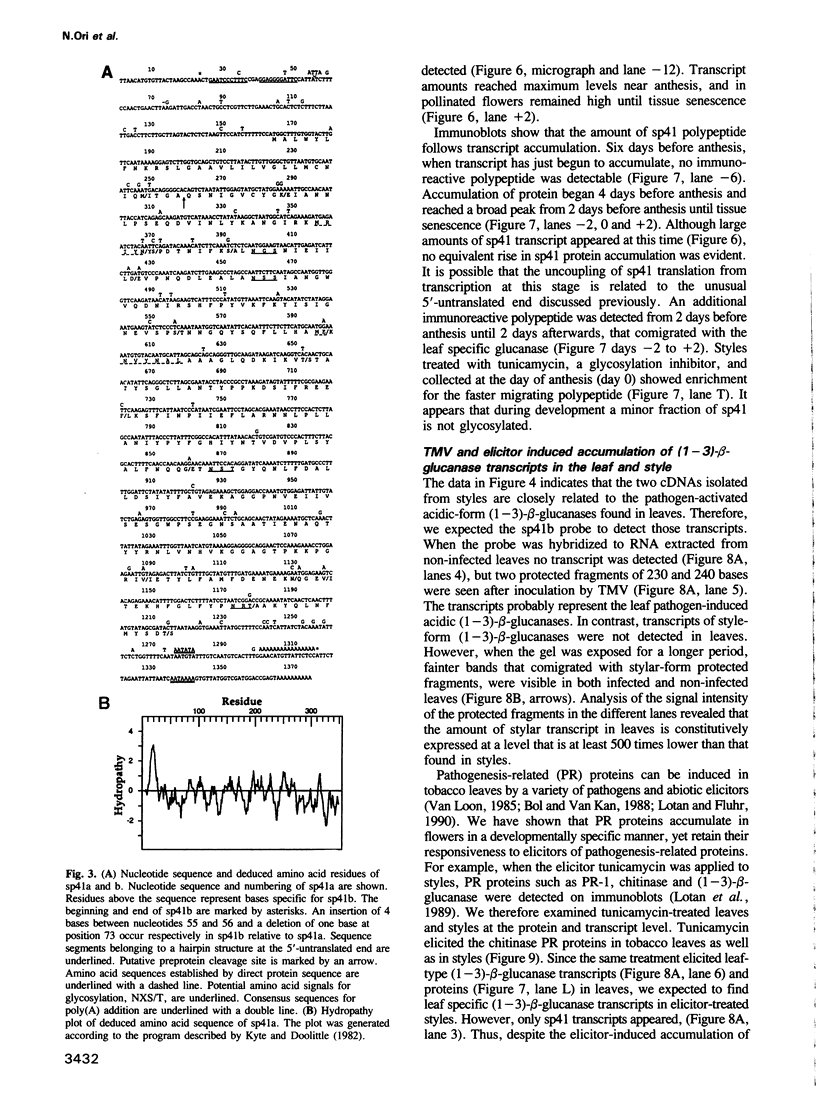
A novel stylar-specific glycosylated protein, sp41, was characterized. Sp41 constitutes greater than 12% of the transmitting tract tissue soluble proteins and is mainly localized in the extracellular matrix. Two cDNA clones corresponding to sp41 mRNA were isolated and sequenced. The decoded sequences are, respectively, 80% and 49% homologous to acidic and basic pathogen-induced (1-3)-beta-glucanases of the leaf. Thus a subfamily of (1-3)-beta-glucanase pathogenesis-related (PR) proteins constitutes one of the major stylar matrix proteins. The accumulation of sp41 transcripts in normally developing and elicitor-treated styles and leaves was followed using an RNase protection assay. During development sp41 transcript accumulation starts well after carpel differentiation. It is first detected in styles at 8 days before anthesis. The maximal level of accumulation is reached during anthesis. Elicitor-treated styles do not accumulate the leaf-type (1-3)-beta-glucanase transcript, although they retain the capacity to synthesize leaf-type pathogenesis-related proteins such as the pathogen-induced acidic chitinase. The developmental regulation of sp41 expression points to a role for them in the normal processes of flowering and reproductive physiology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Podell D. N. Pyroglutamic acid. Non-metabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol Cell Biochem. 1981 Aug 11;38(Spec No)(Pt 1):181–190. doi: 10.1007/BF00235695. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Bol J. F., van Kan J. A. The synthesis and possible functions of virus-induced proteins in plants. Microbiol Sci. 1988 Feb;5(2):47–52. [PubMed] [Google Scholar]
- Broothaerts W. J., van Laere A., Witters R., Préaux G., Decock B., van Damme J., Vendrig J. C. Purification and N-terminal sequencing of style glycoproteins associated with self-incompatibility in Petunia hybrida. Plant Mol Biol. 1990 Jan;14(1):93–102. doi: 10.1007/BF00015658. [DOI] [PubMed] [Google Scholar]
- Bulcke M. V., Bauw G., Castresana C., Van Montagu M., Vandekerckhove J. Characterization of vacuolar and extracellular beta(1,3)-glucanases of tobacco: Evidence for a strictly compartmentalized plant defense system. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2673–2677. doi: 10.1073/pnas.86.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish E. C., Anderson M. A., Clarke A. E. Molecular aspects of fertilization in flowering plants. Annu Rev Cell Biol. 1988;4:209–228. doi: 10.1146/annurev.cb.04.110188.001233. [DOI] [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Kornblihtt A. R., Thiery J. P. The role of fibronectins in embryonic cell migrations. Trends Genet. 1988 Jul;4(7):198–203. doi: 10.1016/0168-9525(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Fincher G. B., Lock P. A., Morgan M. M., Lingelbach K., Wettenhall R. E., Mercer J. F., Brandt A., Thomsen K. K. Primary structure of the (1-->3,1-->4)-beta-D-glucan 4-glucohydrolase from barley aleurone. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2081–2085. doi: 10.1073/pnas.83.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gasser C. S., Budelier K. A., Smith A. G., Shah D. M., Fraley R. T. Isolation of Tissue-Specific cDNAs from Tomato Pistils. Plant Cell. 1989 Jan;1(1):15–24. doi: 10.1105/tpc.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. D., Hamilton D. A., Travis J. L., Bashe D. M., Mascarenhas J. P. Characterization of a pollen-specific cDNA clone from Zea mays and its expression. Plant Cell. 1989 Feb;1(2):173–179. doi: 10.1105/tpc.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høj P. B., Hartman D. J., Morrice N. A., Doan D. N., Fincher G. B. Purification of (1-->3)-beta-glucan endohydrolase isoenzyme II from germinated barley and determination of its primary structure from a cDNA clone. Plant Mol Biol. 1989 Jul;13(1):31–42. doi: 10.1007/BF00027333. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Rodriguez E. B., Stick R. V., Stone B. A. Differences in active site structure in a family of beta-glucan endohydrolases deduced from the kinetics of inactivation by epoxyalkyl beta-oligoglucosides. J Biol Chem. 1989 Mar 25;264(9):4939–4947. [PubMed] [Google Scholar]
- Jofuku K. D., Goldberg R. B. Kunitz trypsin inhibitor genes are differentially expressed during the soybean life cycle and in transformed tobacco plants. Plant Cell. 1989 Nov;1(11):1079–1093. doi: 10.1105/tpc.1.11.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Tokuyasu K. T., Dutton A. H., Singer S. J. An improved procedure for immunoelectron microscopy: ultrathin plastic embedding of immunolabeled ultrathin frozen sections. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5744–5747. doi: 10.1073/pnas.81.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotan T., Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990 Jun;93(2):811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T., Ori N., Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell. 1989 Sep;1(9):881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. A., Frenz D. A., Tomasek J. J., Rabuzzi D. D. Matrix-driven translocation of cells and nonliving particles. Science. 1985 May 17;228(4701):885–889. doi: 10.1126/science.4001925. [DOI] [PubMed] [Google Scholar]
- Rocha-Sosa M., Sonnewald U., Frommer W., Stratmann M., Schell J., Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 1989 Jan;8(1):23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. C., Lord E. M. Directed movement of latex particles in the gynoecia of three species of flowering plants. Science. 1989 Mar 24;243(4898):1606–1608. doi: 10.1126/science.243.4898.1606. [DOI] [PubMed] [Google Scholar]
- Shinshi H., Wenzler H., Neuhaus J. M., Felix G., Hofsteenge J., Meins F. Evidence for N- and C-terminal processing of a plant defense-related enzyme: Primary structure of tobacco prepro-beta-1,3-glucanase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5541–5545. doi: 10.1073/pnas.85.15.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Wu T., Ray A. Adaptors, linkers, and methylation. Methods Enzymol. 1987;152:343–349. doi: 10.1016/0076-6879(87)52041-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]