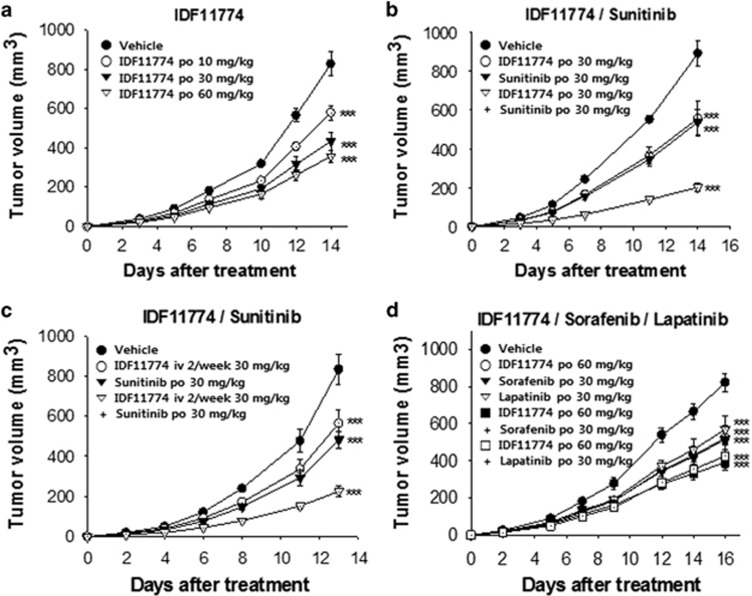
Figure 5.
Antitumor efficacy of IDF-11774 in HCT116 xenograft models. Graphic depictions of tumor size in xenograft model mice in the presence or absence of (a) IDF-11774 (per oral (p.o.), 10, 30, or 60 mg/kg, once a day (q.d.)), (b) IDF-11774 and sunitinib (p.o., 30 mg/kg each, q.d.), (c) IDF-11774 (intranvenously (i.v.), 2/wk; 30 mg/kg, q.d.) and sunitinib (p.o., 30 mg/kg, q.d.), or (d) IDF-11774 (p.o., 60 mg/kg, q.d.) and sorafenib or lapatinib (p.o., 30 mg/kg each, q.d.). Data are presented as the means and standard deviations of the results from five independent experiments; ***P<0.001, compared with vehicle control