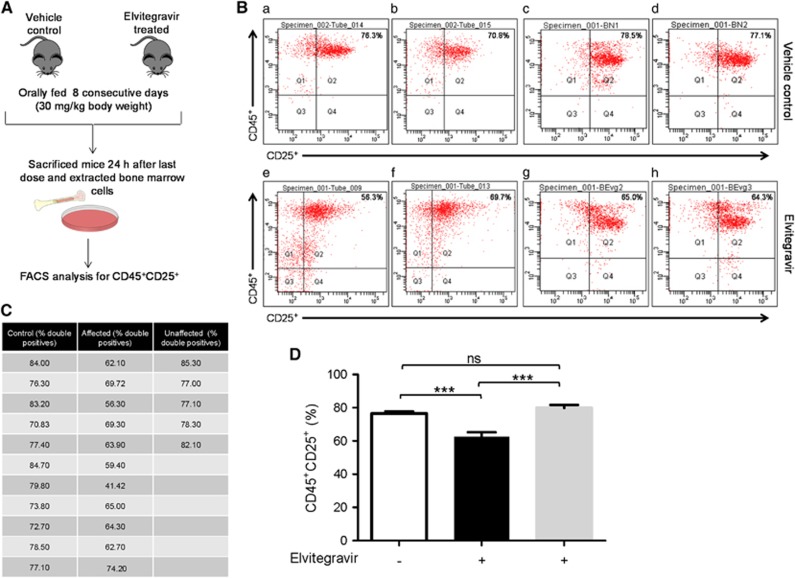
Figure 8.
Evaluation of effect of Elvitegravir on B cells progression in mice by FACS analysis. (A) Schematic representation of steps involved during the in vivo experiment. Balb/c mice (vehicle control and Elvitegravir-treated) were fed with Elvitegravir (8 days; 30 mg/kg). Mice were sacrificed and bone marrow cells were collected, stained with CD45, CD25 surface markers and FACS analysed. (B) Representative FACS dot plots of CD45+CD25+ cells from vehicle control and Elvitegravir-treated mice are shown. Two mice each from independent batches of vehicle control (a,b batch I, c,d batch II) and Elvitegravir-treated groups (e,f batch I, g,h batch II) are presented. (C) Table showing percentage of CD45+CD25+ cells obtained following flow cytometric analysis from control (n=11) and Elvitegravir-treated (n=16) mice from all three batches. Mice, from the Elvitegarvir treated group, that are affected (n=11) and unaffected (n=5) by Elvitegravir are shown. (D) Histogram showing CD45+CD25+ B cells. Double-positive B cells detected following FACS analysis from bone marrow cells of vehicle control (white) are depicted in comparison to Elvitegravir-treated group which is divided into affected (black) and unaffected (grey). While vehicle control mice possessed average 78.03% of CD45+CD25+ B cells in bone marrow it was reduced to average 62.5% following Elvitegravir treatment. ~30% mice were insensitive to treatment. P value <0.001