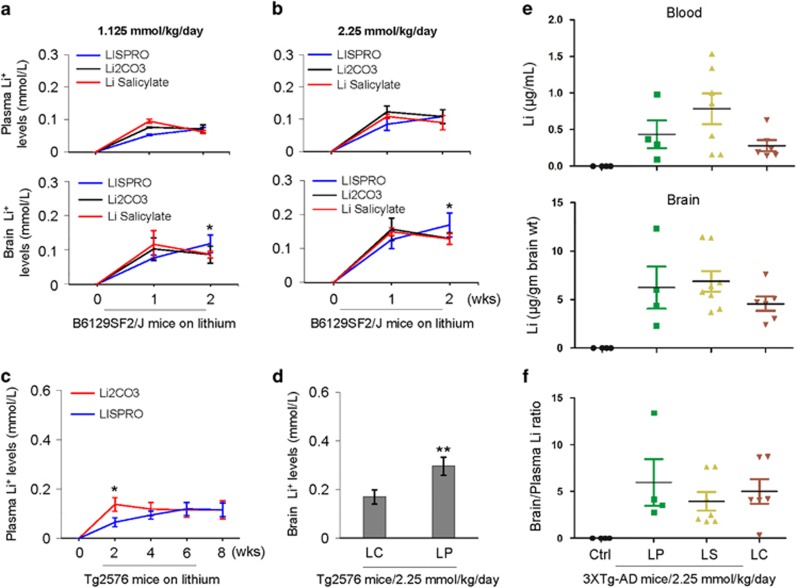
Figure 2.
Plasma and brain lithium pharmacokinetics following chronic oral treatment with LISPRO (LP), lithium salicylate (LS), and Li2CO3 (LC) in B6129SF2/J, Tg2576, and 3XTg-AD mice. (a, b) B6129SF2/J mice (n=2–4 mice/group, male) at 2 months of age were treated for 1 or 2 weeks (wks) with three diets containing LP, LC, or LS, yielding lithium at 1.125 or 2.25 mM/kg/day. (c, d) Tg2576 mice (n=8, 4 female/4 male) at 6 months of age were treated for 8 weeks with two diets containing LP or LC, yielding lithium at 2.25 mM/kg/day. (e, f) Further, 3XTg-AD female mice (n=4–8 mice/group) at 5 months of age were treated for 28 weeks with three diets containing LP, LS, or LC, yielding lithium at 2.25 mM/kg/day, or normal mouse chow (Teklad 2018). Blood and brain lithium levels were measured using atomic absorption spectroscopy (AAS). Brain over plasma lithium ratio calculated for each individual 3XTg-AD mouse (f). All mice received normal drinking water and chow ad libitum. Statistical analysis was carried out using ANOVA with post analysis with Fisher’s LSD test (*P<0.05; **P<0.01). There was no significant difference in plasma or brain lithium levels between LC- and LS-treated B6129SF2/J mice (P>0.05). There was no detectable lithium in plasma and brain homogenates in control Teklad 2018 diet-fed B6129SF2/J, Tg2576, and 3XTg-AD mice (Ctrl, data not shown)