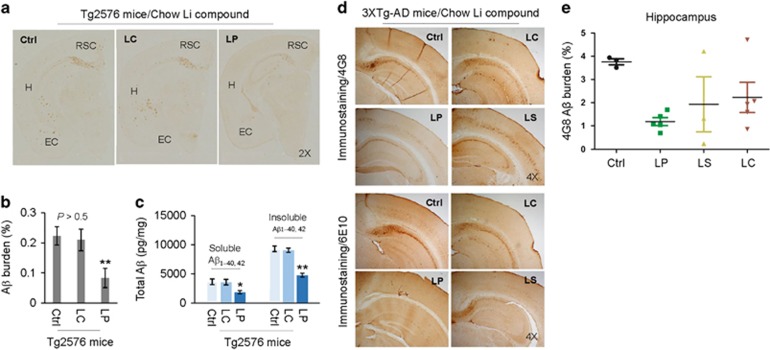
Figure 3.
Oral LP treatment reduces β-amyloid pathology in Tg2576 and 3XTg-AD mice. Tg2576 mice at 8 months of age (n=9; 5 male/4 female) and 3XTg-AD female mice at 5 months of age (n=4–8 mice/group) were treated for 8 or 28 weeks, respectively, with diets containing LP, LS, or LC, yielding lithium at 2.25 mM/kg/day, or normal mouse chow as indicated. These dosages were chosen to give brain lithium concentrations of 0.25–0.50 mM, which fall in a range of clinical therapy for AD.7, 15 All mice received chow and normal drinking water ad libitum. Mouse brain tissue sections and homogenates were prepared from each mouse after treatment. (a, d) Half-brain coronal sections were analyzed by anti-Aβ antibody (4G8) staining. (b,e) Percentage of 4G8 positive plaques (mean±S.E.M.) was quantified by image analysis as described previously.59, 60 (c) Total soluble and insoluble Aβ1–40, 42 peptides from homogenates were analyzed by ELISA and represented as picograms of Aβ peptides per mg of total protein. LP but not LC treatment markedly reduced total soluble and insoluble Aβ1–40,42 levels. A t-test for two samples and one-way ANOVA test for multiple independent samples revealed significant differences between LP and LC (b,c) and among LP, LS, and LC compared with control diet (Ctrl) (e, *P<0.05, **P<0.01). There was no notable or significant difference in both 4G8 positive Aβ plaques and cerebral soluble/insoluble Aβ1–40,42 levels in brain sections and homogenates between LC-treated and control Teklad 2018 diet-fed Tg2576 mice (Ctrl, P>0.05)