Fig. 4. A18 inhibits TH17 cell–induced, but not TH2 cell–induced, airway inflammation in mice.
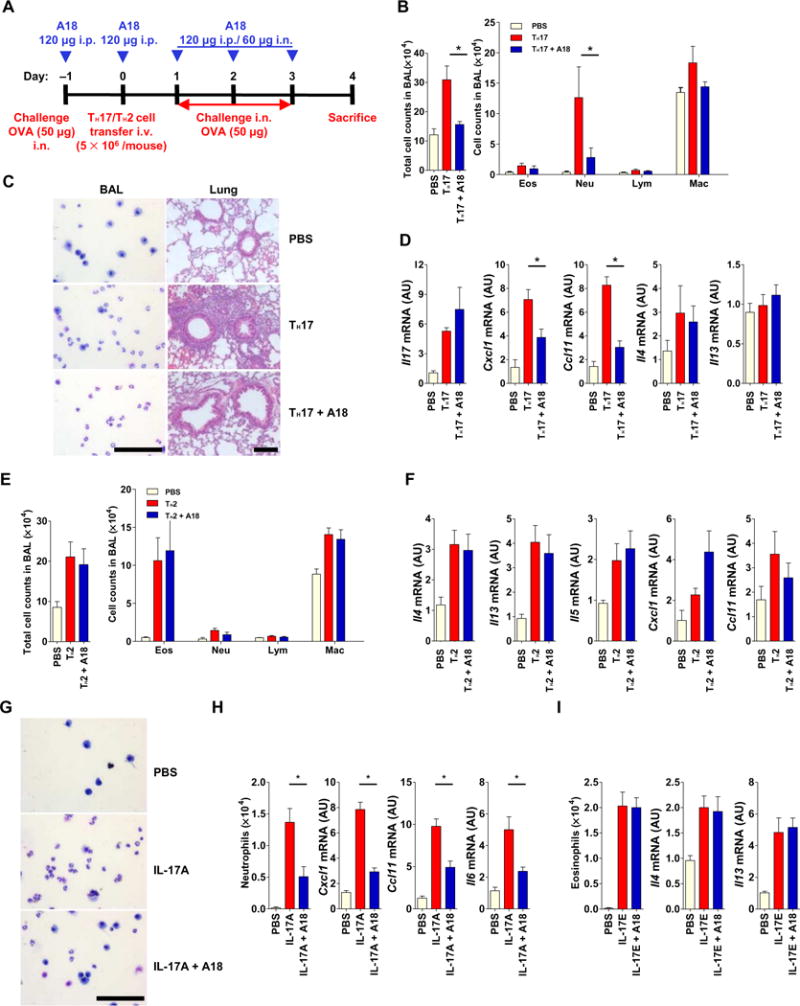
(A) OVA323–339-specific TH17 or TH2 cells were adoptively transferred (with or without A18) into 8-week-old female C57BL/6J mice (n = 5 mice per treatment group), which were treated as indicated to induce antigen-specific airway inflammation. Each TH17 cell– or TH2 cell–based experiment was performed three times with its own PBS control. Data are means ± SEM of three independent experiments. (B to D) When the mice described in (A) that received the TH17 cells were sacrificed, the total and indicated immune cell counts in the bronchoalveolar lavage (BAL) were quantified (B), representative BAL cells were prepared by cytospin and lung tissue was subjected to H&E staining (C), and the relative abundances of the indicated mRNAs isolated from lung tissue were determined by RT-PCR analysis (D). (E and F) When the mice described in (A) that received the TH2 cells were sacrificed, the total and indicated immune cell counts in the BAL were quantified (E). In addition, the relative abundances of the indicated mRNAs were determined by RT-PCR analysis (F). (G to I) IL-17A–induced neutrophilic and IL-17E–induced eosinophilic airway inflammation in the 8-week-old BALB/cJ mice (n = 5 mice per treatment group) with or without A18. (G) Representative BAL cytospin preparations from the mice were analyzed. (H and I) The numbers of neutrophils and eosinophils were counted, and the relative abundances of the indicated mRNAs isolated from lung tissue were determined by RT-PCR analysis. Each IL-17A– or IL-17E–based experiment was performed three times with its own PBS control. Data are means ± SEM of three independent experiments. *P < 0.05. Scale bars, 100 μm. i.p., intraperitoneally; i.v., intravenously; i.n., intranasally.
