Abstract
Introduction
Re-operation is advised for patients with T1b or greater incidental gallbladder cancer (GBCA). The presence of residual disease (RD) impacts resectability, chemotherapy, and survival. This study created a preoperative model to predict RD at re-operation.
Methods
Patients with re-operation for incidental GBCA from 1992–2015 were included. The relationship between pathology data from initial cholecystectomy and RD at re-operation was assessed with logistic regression and classification and regression tree (CART) analysis.
Results
254 patients were included and 188 underwent definitive re-resection (74.0%). Distant RD was identified in 69 (27.2%) patients and locoregional only RD in 82 (32.3%). On multivariate analysis, T3 (OR 22.7, 95% CI:5.5–94.4) and poorly differentiated tumors (OR 4.3, 95% CI:1.4–13.3) were associated with RD (p<0.001–0.012). AUC of multivariate model was 0.78 (95% CI:0.72–0.83). CART analysis split patients into groups based on percentage with RD: 87% RD with T3, 67% RD with T1b/T2 and poorly differentiated, and 35% RD with T1b/T2 and well/moderate differentiated tumors.
Conclusion
Based on T stage and grade from cholecystectomy, this study developed a model for predicting RD at re-operation in incidental GBCA. This model delineates patient groups with variable percentages of RD and could be used to stratify high-risk patients for prospective trials.
Keywords: incidental gallbladder carcinoma, GBCA, residual disease
Introduction
Incidental gallbladder cancer is diagnosed following cholecystectomy for presumed benign biliary disease. It is a rare malignancy with a variable prognosis based on the extent of tumor invasion (1). In patients with invasive tumors greater than T1a (T1b, T2, T3), re-exploration and definitive resection is recommended when there is no evidence of disseminated metastases (2–5). Definitive re-operation includes resection of hepatic segments 4 and 5 and portal lymphadenectomy; however, to obtain tumor clearance, resection may also include major hepatectomy, bile duct excision, or extra-organ resection (6).
Previous studies have found that survival following re-resection is associated with the depth of tumor invasion (T stage) and residual tumor (3). The likelihood of finding residual disease (RD) also increases with T Stage, and RD appears to be a strong determinant of overall prognosis (3, 7–9). Involvement of regional lymph nodes, while also prognostic, is not routinely available with the cholecystectomy specimen and unknown until re-operation and staging (10). Utilizing only factors available from the pathology report from the original cholecystectomy, a recent study found univariate associations between T stage, grade, lymphovascular invasion (LVI), and perineural invasion (PNI) with loco-regional residual disease, distant residual disease and overall survival (11). Preoperative prediction of residual disease has the potential to impact patient management and selection for modified treatment strategies.
Gallbladder cancer has a propensity for early recurrence at local and distant sites (12). Therefore, one of the clinical challenges in incidental gallbladder cancer is predicting the patients that will have residual disease at re-exploration and early progression following surgery. Identification of high-risk patient populations with residual disease may introduce discussions regarding treatment with neoadjuvant chemotherapy (12). This treatment strategy is employed in other aggressive malignancies such as gastric cancer, and would improve patient selection and observation of disease biology prior to attempted re-resection (13, 14). Patients with progression on standard chemotherapy could be spared an operation focused on local control and staging.
Due to the novelty and clinical significance of a prognostic model for incidental gallbladder cancer, this concept warranted exploration within a large surgical series. Furthermore, additional factors such as margins may improve upon the predictive accuracy for residual disease. The aim of this project was to create a preoperative model to predict residual disease status at re-operation in incidental gallbladder cancer.
Methods
Patients and Data Collected
Approval was obtained from the Institutional Review Board for waiver of informed consent. All patients evaluated by a hepatopancreatobiliary surgeon at Memorial Sloan Kettering Cancer Center (MSKCC) were entered into a prospectively-maintained database. Patients with the diagnosis of gallbladder cancer from 1992 to 2015 were included. Two hundred and eighty-eight patients underwent re-operation for incidentally discovered GBCA. Patients were excluded if definitive surgery was performed at another institution or undertaken for strictly palliative reasons (14/288), had a deviation from the expected clinical course with re-operation greater than 1 year following cholecystectomy (3/288), had T1a/Tis or unknown T stage or grade (11/288), or neoadjuvant chemotherapy (6/288). The remaining patients (n=254) formed the study population. Additional demographic, clinicopathologic, and operative data for analysis were collected from the electronic medical record.
Following diagnosis of incidental gallbladder cancer after cholecystectomy, patients were referred to our institution. Variables recorded from the operative note and original pathology report were depth of invasion (T Stage), histology, grade, lymphovascular invasion (LVI), perineural invasion (PNI), margin status (cystic duct and liver margins), lymph node included in cholecystectomy specimen, positive lymph node, and perforation or aspiration at cholecystectomy.
Clinical Approach and Re-operation
Patients’ care was discussed at a multi-disciplinary disease management conference that combined surgeons, medical oncologists, and radiologists. The institutional approach to incidental gallbladder cancer has been previously reported (6, 8, 10, 15). In brief, patients with no evidence of distant disease were selected for re-operation when the depth of invasion was at least T1b. Laparoscopy was used selectively before laparotomy in clinical situations with concern for metastatic disease. When the patient appeared to have localized disease without evidence of distant metastases, laparotomy was performed. Laparoscopic and robotic resections were also performed on selected patients. Surgeons mobilized and palpated the liver, duodenum, pancreatic head, and retroperitoneum. They performed ultrasonography of the liver to evaluate for discontinuous hepatic metastases or involvement of major vasculature. Frozen section biopsies were taken of any suspicious liver, nodal or peritoneal lesions. Patients were generally considered unresectable if there were peritoneal metastases, discontinuous liver metastases, or N2 lymph nodes (outside porta hepatis). Patients with unresectable disease had various procedures performed at the time of surgery including open or laparoscopic biopsy, palliative cholecystectomy, and palliative biliary or enteric bypass. For analysis, these procedures were classified as non-definitive resections.
The extent of definitive resection was determined by the goal of obtaining a negative margin. Patients had segment 4 and 5 resection, major hepatectomy, or extended hepatectomy as appropriate. Lymphadenectomy was performed in the majority of cases and included nodal tissue in the porta hepatis and along the common hepatic artery. The institutional approach to bile duct resection and port site excision varied over the study period. Currently, bile duct resection is not routine and performed only when necessary to obtain negative margins (6). Port site excision was undertaken according to surgeon preference. In recent years, port site excision is no longer routinely practiced at our institution (16).
Locoregional residual disease was defined as any residual disease in the gallbladder fossa, portal or peripancreatic lymph nodes or bile duct. Distant residual disease was classified as discontinuous liver metastases or tumor deposits in the peritoneum or abdominal wall. In situations of uncertain residual disease at exploration, partial hepatic resection was performed and the pathology report served as the final determinant. Residual disease as our primary outcome was classified as any locoregional or distant residual disease.
Statistical Analysis
Demographic information, pathologic variables from original cholecystectomy, and re-operative details were described using counts and percentages for categorical variables and medians and ranges for continuous variables. Univariate and multivariate logistic regression was used to assess the relationship between residual disease at re-operation and pathology from incidental cholecystectomy. Variables initially examined included T stage, grade, LVI, PNI, margins, and perforation/aspiration. As this malignancy is diagnosed incidentally and at outside institutions, potentially relevant pathology information was missing for many patients. To determine if the samples with incomplete information were biased, this analysis of prognostic factors included unknown as a separate category. Only unbiased variables significant at p<0.05 were included in the multivariate analysis.
Additionally, classification and regression tree (CART) analysis using the unbiased variables was employed to determine the optimal variable grouping for the prediction of residual disease. The Gini index was used to grow the tree and the minimum leaf size was set at N=10. The tree was optimized using cross-validation (n=10) and cost-complexity pruning (17, 18). Exact 95% confidence intervals were included around the terminal leaf proportion estimates.
Overall survival (OS) was calculated from the time of re-operation until death or last followup. Patients alive at last followup were censored. The relationship between residual disease and OS was modeled using Kaplan Meier plots and assessed with the log rank test. P-values less than 0.05 were considered statistically significant and all analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
Results
Patient Demographics and Follow-Up
Overall, 254 patients formed the study population and were included in the analysis (Figure 1). Median age at surgery of our sample was 67 years (range: 28–90 years) and 171 (67.3%) were female. The majority of patients were white (210/254, 82.7%). Laparoscopic cholecystectomy (196/254, 77.2%) or laparoscopic converted to open cholecystectomy (43/254, 16.9%) were the most common surgical procedures for the initial operation.
Figure 1.
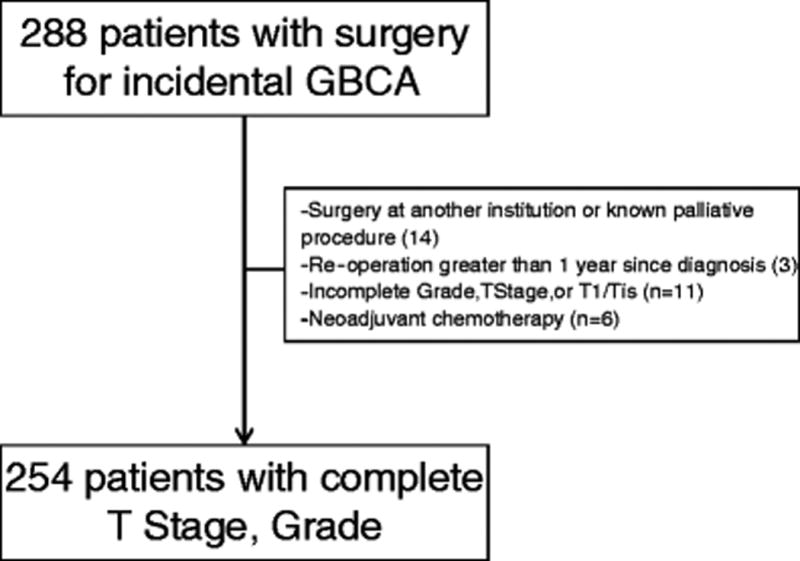
Inclusion flowchart
Pathology Report from Cholecystectomy
Pathology reports from the original cholecystectomy were reviewed. T stage and grade were available for all samples. T stage reported on the surgical specimens was: T1b (17/254, 6.7%), T2 (153/254, 60.2%), and T3 (84/254, 33.1%). Two hundred and thirty-nine patients (94.1%) had histology consistent with adenocarcinoma. Of note, selected variables were missing information for the following proportion of patients: LVI (26/254, 10.2%), PNI (74/254, 29.1%), lymph node status (179/254, 70.5%), margins (42/254, 16.5%), perforation/aspiration (82/254, 32.3%), and gallstones (37/254, 14.6%) (Table 1).
Table 1.
Clinicopathologic factors from cholecystectomy
| N (%) | ||
|---|---|---|
| Cholecystectomy Type | Laparoscopic | 196 (77.2) |
| Laparoscopic to Open | 43 (16.9) | |
| Open | 15 (5.9) | |
| T-Stage | T1b | 17 (6.7) |
| T2 | 153 (60.2) | |
| T3 | 84 (33.1) | |
| Histology | Adenocarcinoma | 239 (94.1) |
| Adenosquamous | 6 (2.4) | |
| Squamous | 2 (0.8) | |
| Neuroendocrine | 4 (1.6) | |
| Sarcoma | 2 (0.8) | |
| Adenosarcoma | 1 (0.4) | |
| Grade | Well Differentiated | 20 (7.9) |
| Moderately Differentiated | 132 (52) | |
| Poorly Differentiated | 102 (40.2) | |
| Lymphovascular Invasion | Unknown | 26 (10.2) |
| No | 116 (45.7) | |
| Yes | 112 (44.1) | |
| Perineural Invasion | Unknown | 74 (29.1) |
| No | 81 (31.9) | |
| Yes | 99 (39) | |
| Positive Lymph Nodes | Unknown | 179 (70.5) |
| No | 34 (13.4) | |
| Yes | 41 (16.1) | |
| Positive Margins | Unknown | 42 (16.5) |
| No | 122 (48) | |
| Yes | 90 (35.4) | |
| Perforation/Aspiration | Unknown | 82 (32.3) |
| No | 95 (37.4) | |
| Yes | 77 (30.3) | |
| Gallstones | Unknown | 37 (14.6) |
| No | 33 (13) | |
| Yes | 184 (72.4) |
Operative Findings and Residual Disease at Re-operation
The median time from initial cholecystectomy to re-operation was 1.5 months (range 0.4–7.4 months). Complete resection was achieved in 74.0% of cases (188/254). Surgical procedures were as listed: segment 4 and 5 resection (154/254, 60.6%), hemihepatectomy (5/254, 2.0%), extended hepatectomy (29/254, 11.4%), and non-definitive resection (66/254, 25.2%). Portal lymphadenectomy was performed in 96.8% of definitive resections (182/188). Among definitive resections, bile duct excision (68/188, 36.2%) and extra-organ resection (8/188, 4.3%) were performed on selected patients (Table 2). Due to changes in practice, approximately one quarter of bile duct resections (15/68, 22.1%) were performed in the interval from 2005–2015, while 78% (53/68) occurred in the preceding years between 1992 and 2004.
Table 2.
Operative findings and procedure at re-operation.
| N (%) | ||
|---|---|---|
| Time until Re-Resection (months) | Median (range) (N=254) | 1.5 (0.4–7.4) |
| Laparoscopy | Yes | 100 (39.4) |
| No | 154 (60.6) | |
| Laparotomy | Yes | 238 (93.7) |
| No | 16 (6.3) | |
| Complete Resection | Yes | 188 (74) |
| No | 66 (26) | |
| Complete Resection Type | NA | 66 (26) |
| Segment 4–5 Resection | 154 (60.6) | |
| Extended Hepatectomy | 29 (11.4) | |
| Hemihepatectomy | 5 (2) | |
| Complete Resection Details | ||
| Bile Duct Resection | Yes | 68 (36.2) |
| No | 120 (63.8) | |
| Lymphadenectomy | Yes | 182 (96.8) |
| No | 6 (3.2) | |
| Extra-Organ Resection | Yes | 8 (4.3) |
| No | 180 (95.7) | |
| Residual Disease | Yes | 151 (59.4) |
| No | 103 (40.6) | |
| Residual Disease Location | None | 103 (40.6) |
| Loco-regional Only | 82 (32.3) | |
| Any Distant | 69 (27.2) |
Residual disease was identified in 151 patients (59.4%), and the absence of residual disease was confirmed by pathology in 103 patients (40.6%). Locoregional only RD was found in 82 patients (32.3%) and included identification of tumor in GB fossa (48/82), bile duct margin (9/82), or lymph nodes (50/82). Among the 69 patients (27.2%) with distant RD, the locations included: peritoneum (50/69), discontinuous liver metastases (14/69), and abdominal wall (8/69).
Univariate and Multivariate Logistic Model for Residual Disease
On univariate analysis, T stage, grade, LVI, margins, PNI, and perforation/aspiration were all associated with residual disease on re-operation. Patients with T3 (OR: 30.97, 95% CI: 7.65–125.44, p<0.001) and T2 (OR: 4.49, 95% CI: 1.24–16.25, p=0.022) tumors had higher odds of RD compared to patients with T1b. Additionally, patients that had poorly differentiated tumors (OR 5.72, 95% CI: 2.06–15.92, p<0.001) had higher odds compared to well differentiated tumors, but no significant difference was seen for moderately differentiated tumors compared to well differentiated tumors (OR: 1.91, 95% CI: 0.72–5.10, p=0.19). Patients with LVI (OR: 2.97, 95% CI: 1.72–5.15, p<0.001), positive margins (OR: 3.38, 95% CI: 1.89–6.07, p<0.001), PNI (OR: 6.25, 95% CI: 3.25–12.02, p<0.001), and perforation or aspiration (OR: 5.02, 95% CI: 2.53–9.95, p<0.001) had higher odds of RD compared to patients without these features. However, patients with unknown LVI (p=0.034), unknown margin status (p<0.001), unknown PNI (p<0.001), and unknown perforation or aspiration (p=0.028) also had higher odds of RD compared to patients without these features (Table 3). These differences between missing and true values indicated a sampling bias such that patients were not missing values at random.
Table 3.
Univariate and multivariate logistic regression for the prediction of residual disease
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p- value |
||
| T-Stage | T3 | 30.97 (7.65–125.44) | <.001 | 22.71 (5.46–94.41) | <.001 |
| T2 | 4.49 (1.24–16.25) | 0.022 | 3.41 (0.91–12.69) | 0.07 | |
| T1b | REF | REF | |||
|
| |||||
| Grade | Poorly Diff | 5.72 (2.06–15.92) | <.001 | 4.28 (1.38–13.29) | 0.012 |
| Moderately Diff | 1.91 (0.72–5.1) | 0.19 | 1.62 (0.54–4.81) | 0.39 | |
| Well Diff | REF | REF | |||
|
| |||||
| Lymphovascular Invasion | Positive | 2.97 (1.72–5.15) | <.001 | -- | |
| Unknown | 2.67 (1.08–6.64) | 0.034 | -- | ||
| Negative | REF | -- | |||
|
| |||||
| Margins | Positive | 3.38 (1.89–6.07) | <.001 | -- | |
| Unknown | 4.77 (2.1–10.83) | <.001 | -- | ||
| Negative | REF | -- | |||
|
| |||||
| Perineural Invasion | Positive | 6.25 (3.25–12.02) | <.001 | -- | |
| Unknown | 3.29 (1.71–6.37) | <.001 | -- | ||
| Negative | REF | -- | |||
|
| |||||
| Perforation/Aspiration | Positive | 5.02 (2.53–9.95) | <.001 | -- | |
| Unknown | 1.96 (1.07–3.56) | 0.028 | -- | ||
| Negative | REF | -- | |||
Therefore, only T stage and grade were included in the multivariate model (Table 3). In the model, T3 patients (OR: 22.71, 95% CI: 5.45–94.41, p<0.001) had significantly higher odds of RD compared to T1b patients. Patients with poorly differentiated histology (OR: 4.28, 95% CI: 1.38–13.29, p=0.012) had significantly higher odds of RD compared to patients with well differentiated histology. T2 stage (p=0.07) and moderately differentiated histology (p=0.39) were not significantly associated with RD in the multivariate model. The AUC of the multivariate model was 0.78 (95% CI: 0.72–0.83).
Classification and Regression Tree Analysis
Based on the findings from the univariate logistic analysis, T stage and grade were considered for the CART analysis. After pruning, both variables remained in the final tree. The first split occurred for T stage, resulting in a leaf for T3 tumors with the probability of having RD at 87% (73/84, 95% CI: 78–93%). T1b and T2 were grouped together and split on grade for the other two leaves. These leaves consisted of poorly differentiated tumors with a 67% (40/60, 95% CI: 53–78%) probability of RD and well and moderately differentiated tumors grouped together with a probability of RD at 35% (38/110, 95%CI: 26–44%) (Figure 2). The cross-validation misclassification rate was 27.7%, with a sensitivity of 74.2% and a specificity of 69.9%.
Figure 2.
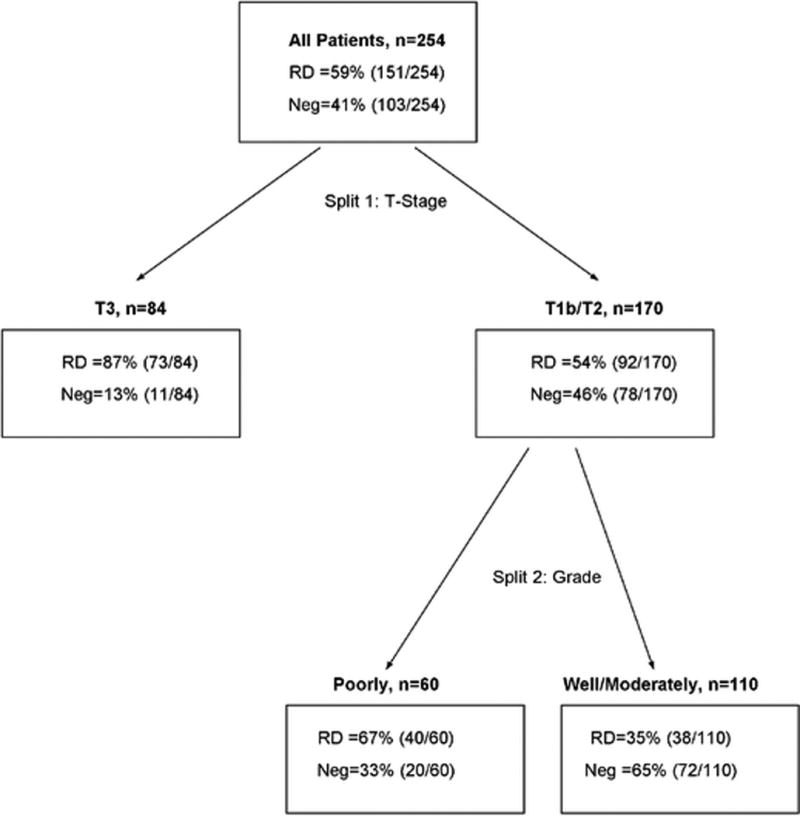
Classification and regression tree (CART) analysis
Overall Survival
As this study focused on pre-operative predictors of RD, all patients were included in survival analysis regardless of final resection status. Patients with residual disease identified at re-operation had significantly worse survival with a median of 17.7 months (95% CI: 15.3–21.1 months) compared to 84.4 months (95% CI: 69.1–145.5) for those patients without residual disease (p<0.001). The 5 year survival estimates were 13.4% (95% CI: 8.6–21.0%) for patients with residual disease and 70.9% (95% CI: 61.1–82.8%) for patients without residual disease (Figure 3).
Figure 3.
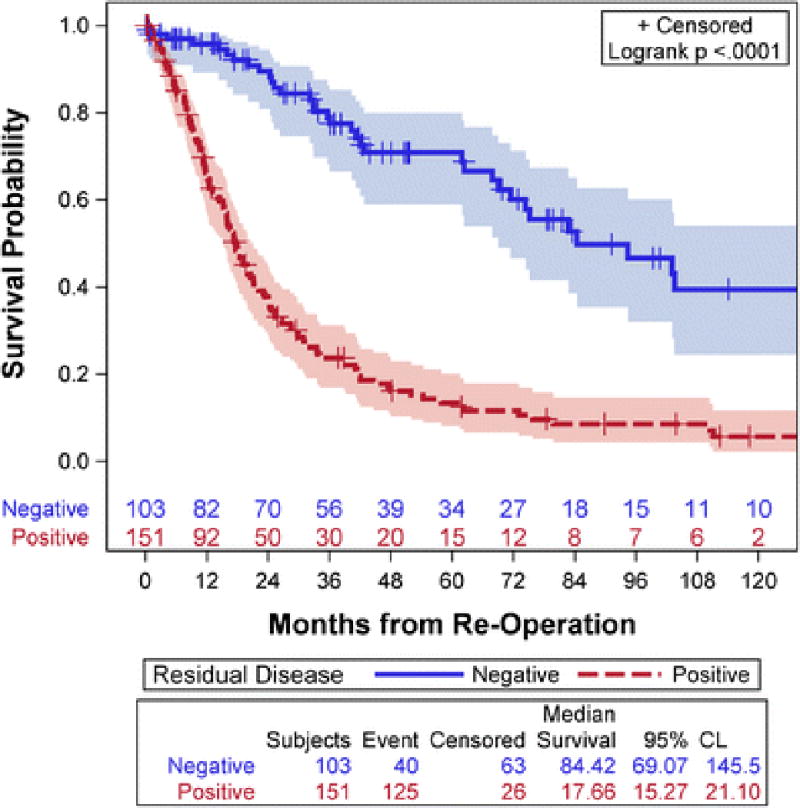
Kaplan Meier plot of overall survival (OS) stratified by Residual Disease
Discussion
Incidental gallbladder cancer is a challenging malignancy with a variable prognosis. The current standard of care is re-resection for invasive tumors T1b or greater (T1b, T2, and T3) and no obvious disseminated disease (5). The rate of residual disease increases with T stage, and previous analyses have demonstrated the presence of RD is associated with poor survival (3, 8). Extrapolating from the results of systemic chemotherapy for locally advanced or unresectable gallbladder cancer, patients with high-risk of RD could be offered novel treatment approaches, such as neoadjuvant chemotherapy, to improve selection of patients for surgery (19). This study utilized factors from the pathology report of cholecystectomy specimens to identify groups of patients with variable percentages of RD in incidental gallbladder cancer.
Our results indicated that T-stage and grade remained predictors of residual disease with an AUC of 0.78 on multivariate analysis, and a sensitivity and specificity of 74% and 70% on cross-validation. The original study concept was to identify prognostic factors in addition to T stage; however, our results demonstrated that the odds of having RD for patients with T3 disease were quite high (OR 22.7) and T-stage was the first split chosen in the CART analysis. Patients with a T3 tumor had an 87% change of having RD at re-operation. These findings reveal the strength of the association between T stage and residual disease. Additionally, grade was available for all patients and created an additional split on the CART analysis. Patients with T1b or T2 and either well or moderately differentiated tumors had only a 35% probability of RD, while those T1b and T2 patients who were poorly differentiated had a 67% probability of RD. As the finding of RD influences the selection of patients for both adjuvant and palliative chemotherapy, high-risk patients could be considered for neoadjuvant chemotherapy prior to surgery. Preoperative prediction of RD allows a potential re-configuration of the treatment timeline. Survival, while an important outcome, was not the focus of this analysis and is influenced by surgical and postoperative factors not known at the time of selection for preoperative chemotherapy. Risk stratification based on RD directly addressed the aim of this project by identifying high-risk groups for a modified treatment strategy.
Other factors, including margins, LVI and PNI, showed an association with RD on univariate analysis. However, our results suggested that patients with missing data were different from patients where this information was available. For instance, patients who had missing PNI status had higher odds of having RD compared to patients who had PNI marked as negative (OR 3.29, p<0.001). Following the work by Rubin, it is now recognized that missing data can only be ignored (i.e. patients with missing data can be left out of the analysis) if it is missing at random, that is if patients with missing data do not constitute a subset with distinctive characteristics (20). Ignoring missing data can result in under or overestimation of our parameter estimates (21, 22). Though it is common to use only complete cases in clinical studies, using complete cases assumes that such missing data are not associated with the outcome. This assumption was violated in our data, and therefore, we did not feel we were able to build a stable prognostic model with these other factors. If this information were to become available, our results could likely change. Therefore, further analyses on these variables were not performed.
Given the possibility that LVI, PNI, and margin data would help us better stratify patients into risk groups, we recommend adherence to standardized pathology reports which include these factors. The College of American Pathologists (CAP) has protocols for examination of cholecystectomy specimens with carcinoma of the gallbladder that includes all of these variables (23). This study forms the foundation for future preoperative models that incorporate additional prognostic variables with minimal unknown data and potential bias. We feel our two factor model provides a simple to follow flowchart with acceptable accuracy. However, with complete data collected in a prospective setting, an appropriately-weighted score with additional factors could be optimized and validated across centers for future implementation.
Ethun et al recently published the Gallbladder Risk Score (GBRS) based on univariate significance of LVI, PNI, T-stage and grade (11). This was the first study to propose a prognostic score to predict RD in incidental gallbladder cancer and prompted investigation of a preoperative model within our data. GBRS groups demonstrated variable percentages of RD and differences in overall survival. This study was encouraging, but suffered from similar missing data limitations. While T stage and grade were available for the majority of patients, LVI and PNI were often missing. Complete data was only available for 88 of 262 patients. The low risk classification only contained 4 patients, so the effectiveness of the full scoring system could not be assessed.
Our analysis reinforces previously described findings about the poor prognosis of residual disease in incidental GBCA (8). The median OS for patients with RD at re-operation was 17 months. However, this analysis was not focused on outcomes after re-operation, as it is the presence of residual disease (either distant RD precluding resection or locoregional only after curative surgery) that often influences the decision to treat patients with chemotherapy. Identification of high-risk patients preoperatively means that modified treatment strategies could be explored in the prospective setting regarding the timing of chemotherapy and re-operation. The aim of this strategy is to better select patients most likely to benefit from surgery or neoadjuvant chemotherapy.
Although focused on delayed staging instead of neoadjuvant chemotherapy, one study from the United Kingdom proposed a treatment strategy that aims to accomplish similar goals (24). Ausania et al. reported the strategy at their institution of re-staging at 3 months and selectively with laparoscopy prior to attempted definitive resection. Forty-nine percent (24/49) of their patients avoided operation when re-staging demonstrated inoperable residual disease. By observing tumor biology, patients were spared the morbidity of re-operation. Our stratification system aims to identify similar high-risk disease subsets, and provides estimates to assist treating oncologists and surgeons in decision-making.
As a single institution that is a tertiary referral center for incidental GBCA, this study reduces variation in operative and clinical treatments across centers; however, this also impacts the generalizability of our findings to other institutions (25, 26). It is possible that the subset of patients referred to our institution is not representative of all incidental GBCA encountered and managed in the community. Our study was retrospective, and as such, subject to inherent selection biases. In addition, no imaging variables were included in this model. Radiographic assessment of residual disease, though imperfect, could also influence the predictive accuracy of RD. However, the subjective nature of imaging following cholecystectomy and selective utilization means that it is vulnerable to the same potential bias as other variables like LVI, PNI, and margins in retrospective analysis. A prospective setting or trial in incidental GBCA, with standard imaging protocols and technique, will provide further information regarding the impact of radiology in predicting RD. Also, although all cases are currently re-reviewed by expert hepatobiliary pathologists in a standard format, older cases do not follow the same guidelines. There is potential bias according to time period in patients that do and do not have full information. Attempts were made to re-review historical cases with missing information, but according to policy the slides had been returned to the original hospital. Therefore a limitation of this study is that the true impact of LVI, PNI, and margin status could not be assessed in our multivariate analysis. Nonetheless, this is the largest, single institution dataset that addresses the ability to predict residual disease in incidental GBCA. Validation of the model in external datasets is necessary for future clinical implementation. We recommend the utilization of a risk stratification system for selection of candidates for neoadjuvant chemotherapy in potential prospective trials.
Conclusion
A model for predicting RD in incidental GBCA was developed using pathology data from the original cholecystectomy. This model delineates patient groups with RD on re-operation, and it could be used to stratify patients for prospective trials of neoadjuvant chemotherapy. Pathologic review of cholecystectomy specimens with gallbladder carcinoma should include these factors in addition to LVI, PNI, and margin status. A prospective setting, that minimizes any unknown variables, would allow development of a more robust model for RD in incidental GBCA.
Acknowledgments
Funding: This work was supported in part by the NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
Disclosure: Authors report no conflicts of interest.
Presentation: This work has not been previously presented.
Author Contributions: All authors met criteria for authorship based on contributions to the manuscript in submission.
References
- 1.Hueman MT, Vollmer CM, Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Annals of surgical oncology. 2009;16(8):2101–15. doi: 10.1245/s10434-009-0538-x. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Annals of surgery. 2000;232(4):557–69. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World journal of surgery. 2011;35(8):1887–97. doi: 10.1007/s00268-011-1134-3. [DOI] [PubMed] [Google Scholar]
- 4.Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Annals of surgery. 2008;247(1):104–8. doi: 10.1097/SLA.0b013e318154bf5d. [DOI] [PubMed] [Google Scholar]
- 5.Aloia TA, Jarufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015;17(8):681–90. doi: 10.1111/hpb.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the Extent of Resection for Adenocarcinoma of the Gallbladder. Annals of surgical oncology. 2009;16(4):806–16. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2007;11(11):1478–86. doi: 10.1007/s11605-007-0309-6. discussion 86-7. [DOI] [PubMed] [Google Scholar]
- 8.Butte JM, Kingham TP, Gönen M, et al. Residual Disease Predicts Outcomes after Definitive Resection for Incidental Gallbladder Cancer. Journal of the American College of Surgeons. 2014;219(3):416–29. doi: 10.1016/j.jamcollsurg.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butte JM, Waugh E, Meneses M, et al. Incidental Gallbladder Cancer: Analysis of Surgical Findings and Survival. Journal of surgical oncology. 2010;102(6):620–5. doi: 10.1002/jso.21681. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Ito K, D'Angelica M, et al. Accurate Staging for Gallbladder Cancer Implications for Surgical Therapy and Pathological Assessment. Annals of surgery. 2011;254(2):320–5. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 11.Ethun CG, Postlewait LM, Le N, et al. A Novel Pathology-Based Preoperative Risk Score to Predict Locoregional Residual and Distant Disease and Survival for Incidental Gallbladder Cancer: A 10-Institution Study from the U.S. Extrahepatic Biliary Malignancy Consortium. Annals of surgical oncology. 2016 doi: 10.1245/s10434-016-5637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689–700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World journal of gastroenterology. 2010;16(44):5621–8. doi: 10.3748/wjg.v16.i44.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Annals of surgical oncology. 2015;22(4):1153–9. doi: 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. Hpb. 2011;13(7):463–72. doi: 10.1111/j.1477-2574.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maker AV, Butte JM, Oxenberg J, et al. Is port site resection necessary in the surgical management of gallbladder cancer? Annals of surgical oncology. 2012;19(2):409–17. doi: 10.1245/s10434-011-1850-9. [DOI] [PubMed] [Google Scholar]
- 17.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. 2. xxii. New York, NY: Springer; 2009. p. 745. [Google Scholar]
- 18.Zhang H, Singer B, Zhang H. Recursive partitioning and applications. 2. xiv. New York: Springer; 2010. p. 259. [Google Scholar]
- 19.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New England journal of medicine. 2010;362(14):1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Inference and Missing Data. Biometrika. 1976;63(3):581–90. [Google Scholar]
- 21.Vach W, Blettner M. Missing data in epidemiologic studies. Encyclopedia of biostatistics. 1998 [Google Scholar]
- 22.Burton A, Altman DG. Missing covariate data within cancer prognostic studies: a review of current reporting and proposed guidelines. British journal of cancer. 2004;91(1):4–8. doi: 10.1038/sj.bjc.6601907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.College of American Patholgists. [cited 2016 September 30, 2016];Cancer Protocols. 2012 Available from: http://www.cap.org/web/hom/resources/cancer-reporting-tools/cancer-protocols.
- 24.Ausania F, Tsirlis T, White SA, et al. Incidental pT2-T3 gallbladder cancer after a cholecystectomy: outcome of staging at 3 months prior to a radical resection. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2013;15(8):633–7. doi: 10.1111/hpb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC), 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) Journal of surgical oncology. 2008;98(7):485–9. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Annals of surgery. 1996;224(5):639–46. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]


