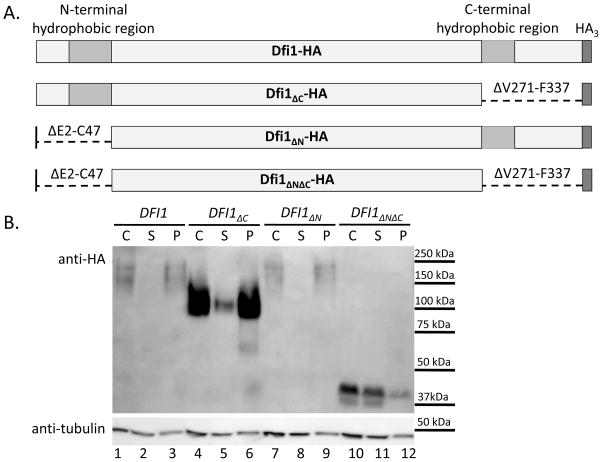
Figure 1. Either hydrophobic region is sufficient for membrane association of Dfi1.
Panel A. Diagrams of Dfi1 protein variants. Diagrams show full-length Dfi1 (Dfi1-HA), Dfi1 lacking the C-terminal hydrophobic region (Dfi1ΔC-HA), Dfi1 lacking the N-terminal hydrophobic region (Dfi1ΔN-HA), or Dfi1 in which the both hydrophobic regions were deleted (Dfi1ΔNΔC-HA). Diagrams are not to scale.
Panel B. Membrane association. Cell lysates of C. albicans strains encoding HA-tagged full-length Dfi1, Dfi1ΔC, Dfi1ΔN, or Dfi1ΔNΔC were made as described in the Materials and Methods. Lysates underwent ultracentrifugation at 300,000g for 20 min to pellet cell membranes. Equal volumes of the crude lysate before ultracentrifugation (C), and the supernatant (S) and pellet (P) resulting from ultracentrifugation, were loaded into each lane, and the resulting membrane was probed for HA (top image) and tubulin (bottom image). This blot is representative of three independent experiments.