Introduction
In this issue of Cancer Prevention Research, Islam and colleagues from the Browning group report that sildenafil (Viagra), an FDA-approved drug prescribed for the treatment of pulmonary arterial hypertension (PAH) and erectile dysfunction (ED), can suppress colitis and tumor development in an inflammation-driven mouse model of colorectal cancer [1]. Sildenafil belongs to a class of drugs, including vardenafil (Levitra) and tadalafil (Cialis) that potently and selectively inhibit the phosphodiesterase (PDE) isozyme, PDE5. PDE5 has a highly specific function to catalyze the hydrolysis of cyclic guanosine monophosphate (cGMP), a critically important signaling molecule capable of regulating multiple aspects of cell physiology. By inhibiting cGMP degradation, PDE5 inhibitors can rapidly increase intracellular cGMP levels to activate cGMP-dependent protein kinases (PKG) and phosphorylate key proteins that regulate diverse cellular functions. Relaxation of smooth muscle cells lining the blood vessels of the pulmonary circulation or the corpus cavernosum of the penis explains the benefits of sildenafil for PAH or ED, respectively, while suppression of cell proliferation and increased mucinous differentiation in the colonic mucosa appear to be responsible for the ability of sildenafil to inhibit colorectal tumorigenesis. The authors also provide evidence that colorectal tumorigenesis is associated with deficiencies in cGMP levels.
Background
Chronic inflammation is a well-known driver of tumorigenesis. Nonsteroidal anti-inflammatory drugs (NSAIDs) have been widely reported to inhibit tumorigenesis in multiple rodent models, while many epidemiological studies have concluded that the long-term use of NSAIDs can significantly reduce the risk of death from colorectal and other cancers in humans. The rationale for evaluating sildenafil in an inflammation-driven mouse model of colorectal cancer stemmed from previous studies reporting chemopreventive activity of sulindac sulfone (exisulind) in a rat model of chemical-induced colon tumorigenesis [2]. Sulindac sulfone is a non-cyclooxygenase (COX) inhibitory metabolite of the NSAID, sulindac that inhibits colon tumor cell proliferation and induces apoptosis by a mechanism reported to involve the inhibition of PDE5 and possibly other cGMP PDE isozymes [3]. An association between potency to inhibit tumor cell proliferation and cGMP PDE activity was also observed for the COX inhibitory sulfide metabolite of sulindac [4, 5]. Sulindac is among the most effective NSAIDs to cause regression of adenomas in familial adenomatous polyposis (FAP) patients [6], but the long-term use of sulindac or other NSAIDs for chemoprevention is not recommended because of gastrointestinal and other toxicities resulting from COX inhibition and the suppression of physiologically important prostaglandins.
Likely because of a structural resemblance to cGMP, sulindac sulfide inhibits cGMP hydrolysis by several PDE isozymes, including PDE2, 3, 5, and 10 at concentrations that inhibit colon tumor cell growth in vitro [7]. PDE5 is the most sensitive PDE isozyme to sulindac sulfide that appears to be an important target based on experiments showing that PDE5 knockdown by siRNA, PDE5 inhibition by small molecules, and cGMP agonists inhibit colon tumor cell growth and that PDE5 is up-regulated in colon adenomas and adenocarcinomas relative to normal mucosa [4, 8]. However, the involvement of additional cGMP PDE isozymes could not be ruled out given that high concentrations of sildenafil exceeding those required to inhibit purified PDE5 are necessary to inhibit colon tumor cell growth whereas the inhibition of additional cGMP PDE isozymes could contribute to its tumor cell growth inhibitory activity. Thus, the in vivo evaluation of sildenafil in a colitis-induced mouse model of colon tumorigenesis effectively tested the hypothesis that PDE5 represents a target for colorectal cancer chemoprevention.
Salient Findings
Experiments in mice described by Browning show that a clinically relevant dosage of sildenafil can inhibit proliferation and induce mucinous differentiation in colonic mucosa. Intestinal damage caused by the irritant, dextran sulfate sodium (DSS) as assessed by barrier function and other indicators of colitis, including weight loss, bleeding, and diarrhea was suppressed by sildenafil. Sildenafil also protected against weight loss and reduced the multiplicity of colon polyps by approximately 50% in mice challenged with DSS and the carcinogen, azoxymethane (AOM). Treatment with sildenafil prior to the induction of inflammation with DSS was as effective as continuous treatment, although starting sildenafil after inducing inflammation was ineffective. The authors also noted a pronounced treatment effect of sildenafil on mucinous differentiation in the normal colon mucosa, which was also observed by other investigators analyzing biopsies from FAP patients treated with exisulind where an inhibitory effect on polyp formation was observed [9]. Interestingly, the reduced proliferation and increased mucus was also observed in polyps of sildenafil-treated mice in the absence of detectable PKG activity. This could be explained by a suppressive effect of sildenafil on myeloid-derived suppressor cells and supported by experiments showing reduced infiltration of myeloid cells, as well as the expression of inflammatory mediators in the treated polyps.
Evidence that colon tumorigenesis is associated with deficiencies in cGMP production is shown by decreased levels of guanylin and guanylate cyclase C in polyps as compared with normal colonic mucosa. While Browning reports that PDE5 mRNA levels were not elevated in polyps relative to normal mucosa, mRNA levels of another cGMP degrading PDE isozyme, PDE10, were strongly induced in polyps, which is consistent with reports by other investigators showing that PDE10 is elevated in human colon adenomas and adenocarcinomas [9]. cGMP may therefore play an important role as a tumor suppressor in the colonic mucosa where intracellular levels are aberrantly low as a result of reduced expression of guanylate cyclase and/or increased expression of PDE10 in neoplastic cells.
Corroboration
A recent publication by Lin and colleagues essentially replicates observations made by Browning using the AOM-DSS mouse model, although certain discrepancies were apparent that will need to be resolved by future research [12]. For example, Lin showed that sildenafil was ineffective if tumors were induced with only AOM, which suggests that the ability of sildenafil to inhibit tumor formation in the AOM-DSS model is closely tied to an anti-inflammatory effect and consistent with an inhibitory effect of sildenafil on inflammatory mediators and infiltrate. On the other hand, Browning shows that sildenafil was effective if administered prior to the induction of inflammation, which suggests an effect primarily during the initiation stage of tumorigenesis, possibly by reduced proliferation and a more resilient barrier function to make the epithelium more resistant to genotoxic stress by AOM. However, sildenafil was not effective if administered after the 1st DSS cycle during the promotion stage of tumorigenesis. In addition, Browning reported that sildenafil decreased cell proliferation, but did not induce apoptosis and observed no change in PDE5 mRNA levels, while Li et al. reported decreased proliferation and increased apoptosis with sildenafil treatment, along with an increase in PDE5 protein levels in tumors relative to normal mucosa. Some of these differences may be attributed to differences in how sildenafil was administered between the two groups. For example, Browning administered sildenafil at a low dose continuously by drinking water, while Lin administered a high dose by multiple intraperitoneal injections.
Interpretations
The preventive activity of sildenafil on polyp formation as observed by Browning could be interpreted as modest given that treatment caused only a 50% inhibition of polyp formation, as well as the inability of sildenafil to inhibit tumor formation if treatment was started during late stages of carcinogenesis. A possible explanation for the modest activity of sildenafil as observed in the AOM-DSS mouse model may be that high expression of a cGMP degrading PDE isozyme other than PDE5 in neoplastic cells could override or compensate for a PDE5 selective inhibitor by maintaining low intracellular cGMP levels. Indeed, the PDE isozyme composition within a given tissue is well known to be an important determinant of sensitivity to PDE isozyme-specific inhibitors. As such, the prominent overexpression of PDE10 relative to PDE5 in polyps compared to normal colonic mucosa may limit the sensitivity to sildenafil treatment or cause resistance. Previous reports that PDE10 is essential for the proliferation of colon tumor cells and that PDE10 inhibitors are highly effective in the APC mouse model colon cancer suggest potential advantages of combining a PDE10 and PDE5 inhibitor [10, 11, 13, 14]. There may also be benefits of combining sildenafil with sulindac to activate cGMP signaling and inhibit COX activity for suppressing both the proliferative and inflammatory components of colorectal cancer progression.
Conclusions
The strength of the Browning publication is the use of a pharmacologically relevant dose of sildenafil to prevent colorectal cancer in a mouse model equivalent to a 70 kg man taking a 35 mg daily dose that could be rapidly translated to human clinical trials. While the efficacy of sildenafil in the AOM-DSS mouse model might be perceived as modest, one cannot predict how sildenafil will perform in humans at risk of developing colorectal cancer, for example, from polyposis syndromes, family history, or inflammatory bowel disease (IBD). This study also supports clinical trials to determine potential benefits of combining sildenafil with sulindac for colorectal cancer chemoprevention in individuals with IBD to suppress tumor cell proliferation and inflammation. In addition, the observations encourage epidemiological studies to examine a potential relationship between the use of PDE5 inhibitors and the incidence of or risk of death from colorectal cancer in the general population. Since sustained plasma levels of a PDE5 inhibitor may be optimal for suppressing inflammation and tumor cell proliferation, it would be interesting to determine if long-acting PDE5 inhibitors having an extended half-life (e.g. tadalafil) will out-perform sildenafil. Finally, the observations support further exploratory research into a potential role of PDE10 in colorectal carcinogenesis and the development of PDE10 inhibitors for chemoprevention in individuals with polyposis syndromes at a high risk of developing colorectal cancer, especially with the possibility of treating sessile polyps that may be missed during colonoscopy.
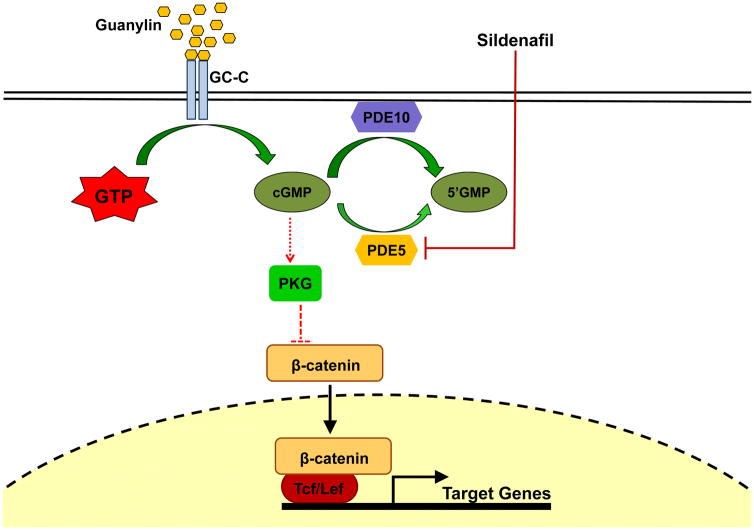
Figure 1.
Scheme showing PDE5/cGMP regulation of β-catenin transcriptional activity. Red dotted lines indicate the effect of sildenafil. The large size arrow representing degradation of cGMP by PDE10 signifies a potential mechanism of resistance to sildenafil.
Footnotes
Disclosure of Potential Conflicts of Interest
G.A. Piazza is a founder of PDEi Pharmaceuticals Inc. and co-founder of ADT Pharmaceuticals Inc.
References
- 1.Islam, et al. Current issue [Google Scholar]
- 2.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Bogert C, Guillen JM, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis without reducing prostaglandin levels. Cancer Research. 1997;57:2909–2916. [PubMed] [Google Scholar]
- 3.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, et al. Exisulind induced apoptosis involves cGMP PDE Inhibition, PKG activation, and attenuated β-catenin. Cancer Res. 2000;60:3338– 3342. [PubMed] [Google Scholar]
- 4.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, et al. Colon tumor cell growth inhibitory activity of sulindac sulfide and other NSAIDs is associated with PDE5 inhibition. Cancer Prev Res. 2010;3:1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurpinar E, Grizzle WE, Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res. 2014;20:1104–13. doi: 10.1158/1078-0432.CCR-13-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 7.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, et al. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res. 2012;5:822–33. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, et al. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/beta-catenin signaling. Mol Cancer Ther. 2013;12:1848–59. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoner GD, Budd GT, Ganapathi R, DeYoung B, Kresty LA, Nitert M, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv Exp Med Biol. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Lee K, Xi Y, Zhu B, Gary BD, Ramírez-Alcántara V, et al. Phosphodiesterase 10A: a novel target for selective inhibition of colon tumor cell growth and β-catenin-dependent TCF transcriptional activity. Oncogene. 2015;34:1499–1509. doi: 10.1038/onc.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Lindsey AS, Li N, Gary B, Andrews J, Keeton AB, Piazza GA. β-catenin nuclear translocation in colorectal cancer cells is suppressed by PDE10A inhibition, cGMP elevation, and activation of PKG. Oncotarget. 2015;7:5353–5365. doi: 10.18632/oncotarget.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, et al. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res. 2017;7:41–52. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KJ, Chen X, Valiyaveettil J, Lindsey AS, Andrews J, Ramirez-Alcantara V, et al. Novel non-COX inhibitory sulindac derivative with β-catenin suppressing activity reduces the formation of colorectal adenomas and adenocarcinomas in the APC+/min-FCCC mouse model. Proceedings of the AACR. 2017:5243. [Google Scholar]
- 14.Li N, Chen X, Zhu B, Ramírez-Alcántara V, Canzoneri JC, Lee K, et al. Suppression of β-catenin/TCF transcriptional activity and colon tumor cell growth by dual inhibition of PDE5 and 10. Oncotarget. 2015;6:27403–27415. doi: 10.18632/oncotarget.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]