Figure 4.
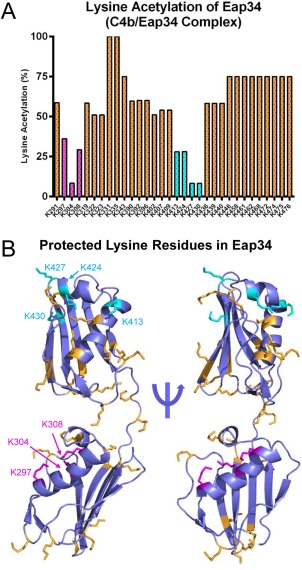
Specific lysine residues in Eap34 experience changes in solvent accessibility upon C4b binding. The solvent accessibility of Eap34 lysine residues was examined in the absence or presence of C4b through a chemical footprinting approach. A chymotrypsin digestion coverage map was first obtained for Eap34 as described in the Materials and Methods section, and changes in m/z of various peptides were characterized in an identical sample that had been treated with NHS‐Ac. Alterations in these acetylation modification patterns were studied for a pre‐formed C4b/Eap34 complex. The results of four independent experiments were merged to arrive at a total number of observations for each individual lysine residue. (A) Relative protection status of each observable Eap34 lysine in samples of C4b/Eap34. Positions K297, K304, and K308 of Eap3 (magenta), and K413, K424, K427, and K430 in Eap4 (cyan) were acetylated in less than 33% of all observations, implying they were less solvent‐accessible than other Eap34 lysines (orange) when bound to C4b. (B) Structural context of the lysine residues in Eap34. All lysine sidechains are rendered in ball‐and‐stick convention. Residues that were readily acetylated in the experiment described above are colored orange, while those identified as protected from acetylation when bound to C4b are colored as magenta (Eap3) and cyan (Eap4), respectively.
