Abstract
A pandemic of metabolic diseases, consisting of type 2 diabetes, nonalcoholic fatty liver disease, and obesity, has imposed critical challenges for societies worldwide, prompting investigation of underlying mechanisms and exploration of low-cost and effective treatment. In this report, we demonstrate that metabolic disorders in mice generated by feeding with a high-fat diet without dietary vitamin D can be prevented by oral administration of polycationic amine resin. Oral administration of cholestyramine, but not the control uncharged polystyrene, was able to sequester negatively charged bacterial endotoxin in the gut, leading to 1) reduced plasma endotoxin levels, 2) resolved systemic inflammation and hepatic steatohepatitis, and 3) improved insulin sensitivity. Gut dysbiosis, characterized as an increase of the phylum Firmicutes and a decrease of Bacteroidetes and Akkermansia muciniphila, was fully corrected by cholestyramine, indicating that the negatively charged components in the gut are critical for the dysbiosis. Furthermore, fecal bacteria transplant, derived from cholestyramine-treated animals, was sufficient to antagonize the metabolic disorders of the recipient mice. These results indicate that the negatively charged components produced by dysbiosis are critical for biogenesis of metabolic disorders and also show a potential application of cationic polystyrene to treat metabolic disorders through promoting gut eubiosis.
Introduction
Metabolic syndrome (MetS), in association with obesity, fatty liver diseases, and type 2 diabetes, has imposed great health challenges and an enormous economic burden on societies worldwide (1,2). A key mechanism for MetS development is insulin resistance, which is often generated by chronic inflammation that attenuates insulin signal transduction pathways, resulting in hyperglycemia and hyperlipidemia (3,4). Gut dysbiosis, showing increased Firmicutes and reduction of Bacteroidetes, is critical for the biogenesis of MetS and obesity (5–7). Bacteroidetes are Gram-negative microbes, and their death may generate endotoxin, a highly potent inflammatory component in the gut. On the other hand, simple hepatic steatosis is benign, but ∼20% of the population with simple steatosis may further progress to nonalcoholic steatohepatitis (NASH), characterized by hepatic inflammation and liver fibrosis (8,9). To explain the transition from simple steatosis to NASH, a “two-hit theory” was proposed, suggesting that a second hit, such as viral infection/xenobiotic exposure or others may drive the pathophysiological progression (10).
Epidemic survey indicates association of vitamin D deficiency with chronic liver diseases, obesity, and MetS (11–13). Through animal models, we previously demonstrated that vitamin D deficiency acts as a “second hit” to exacerbate the impact of high-fat diet, leading to robust metabolic disorders with features of NASH (14). Recently, we showed that vitamin D signaling through the induction of Paneth cell–specific α-defensins maintains gut eubiosis to antagonize MetS (15). Given the nature of positive charges by the secretory form of α-defensins, we reasoned that applying positively charged resins might have similar impacts.
Research Design and Methods
Animal Treatment and Measurement
C57BL/6J mice (male, 4–6 weeks old) were fed either control chow (AIN93, containing 10–12% calories from fat and 1,000 units/kg vitamin D3; HFK Bioscience) or vitamin D–deficient high-fat diet (HD, 60% calories from fat and without vitamin D supplement). After 10 weeks of feeding, the mice fed with HD were divided into three groups (n = 10) and subjected to 1) no treatment (HD), 2) cholestyramine added to HD diet at 3% (weight for weight) (HD+CHOL), or 3) polystyrene treatment (HD+PS), for an additional 12 weeks. For fecal bacteria transplant (FBT), fresh donor feces from cholestyramine or polystyrene treatments were collected. After washing to remove the resins, ∼4 × 108 colony-forming units of bacteria in 0.1 mL suspension were administered by gavage to the recipient mice that had been fed with either control chow or HD (n = 8–12) for 20 weeks. FBT was repeated once a week for a consecutive 8 weeks. Changing microbiota in the feces and ileal lumen content was monitored by 16S rDNA-qPCR analysis. The animal protocol followed the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. The protocols for intraperitoneal glucose tolerance test (IPGTT) and HOMA of insulin resistance (HOMA-IR), fasting glucose, intestinal permeability, and histological analysis were previously reported (15). A limulus amebocyte extract kit (Chinese Horseshoe Crab Reagent Manufactory, Xiamen, China) was used to detect plasma lipopolysaccharide (LPS) content. Gene expression was determined by qRT-PCR analysis and normalized to that of RPL-19. Primer sequence information used for qPCR analysis is listed (Supplementary Tables 1 and 2).
In Vitro Sequestration of LPS by Cationic Resins
LPS (catalog no. L3012; Sigma-Aldrich) was dissolved in pure water and specific adsorption was recorded at 258 nm. Cholestyramine, polystyrene powder, and Pierce High Capacity Endotoxin Removal Resin (catalog no. 88270; Thermo Fisher Scientific) were mixed with LPS solution. The mixtures were incubated for 1 h. After centrifuging at 500g for 1 min, the supernatant was filtered through a 0.45-micron filter, and adsorption was measured at 258 nm for the unbound LPS. The experiment was repeated three times.
Gut Microbiota Analysis
The work was conducted at Novogene (Beijing, China). In brief, mice were fed and treated with the following four conditions: 1) control chow, 2) HD, 3) HD+CHOL, or 4) HD+PS for 12 weeks (n = 5 for each condition). The fecal microbe DNA was extracted using the stool DNA kit (Omega, China) method. Details can be found in our previous work (15).
Statistical Analysis
Data recording, processing, and calculating were completed using Microsoft Excel 2003 and GraphPad Prism 5.0. Data were plotted as mean ± SEM. Comparisons across groups were assessed using a Student t test or one-way ANOVA, followed by Tukey multiple comparison testing. Statistical significance was performed as *P < 0.05 or **P < 0.01.
Results
Metabolic Disorders in Mice Are Resolved by Oral Administration of Cholestyramine Through Sequestrating Gut Endotoxin
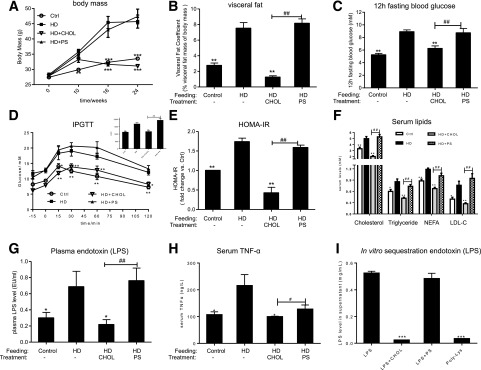
As expected, the mice fed HD gained substantial weight together with increased visceral fat accumulation compared with those fed with control diet (Fig. 1A and B). The mice fed with HD developed glucose intolerance and insulin resistance, as indicated by elevation of fasting blood glucose, abnormal IPGTT, and a high level of HOMA-IR values (Fig. 1C–E). Likewise, dyslipidemia was observed in the HD mice, showing increased levels of serum total cholesterol, triglyceride, nonesterified fatty acids, and LDL cholesterol (LDL-C) (Fig. 1F). Nevertheless, all the features of metabolic disorders were significantly improved with oral administration of cholestyramine (HD+CHOL). On the other hand, uncharged polystyrene beads were without significant impact. Importantly, these results indicated the presence of “negatively charged components” in the gut as causative factors in the insulin resistance and metabolic disorders in the murine model.
Figure 1.
Metabolic disorders in the mice fed with HD are resolved by oral administration of cholestyramine. C57/B6 mice were fed with control chow (Ctrl, AIN93, containing vitamin D3 1,000 IU/kg) or HD (60% calories from fat without vitamin D supplement) for 22 weeks, as a negative and positive control, respectively. For the treatment groups, the mice were initially fed with HD for 10 weeks. Then cholestyramine or polystyrene at 3% (weight for weight) was added in the HD diet, as HD+CHOL and HD+PS, respectively (n = 10). A: Time course of changing body mass (n = 10). B: Visceral fat coefficient, the percentage of visceral fat mass over the body mass. C: Fasting blood glucose (12 h), n = 10. D: IPGTT and area under the curve (AUC) for the mice after 20 weeks of feeding, n = 6. E: HOMA-IR after 20 weeks feeding. F: Serum total cholesterol, serum total triglyceride, serum nonesterified fatty acids (NEFA), and serum LDL-C. G: The levels of plasma endotoxin (LPS), n = 5. EU, endotoxin units. H: The levels of serum TNF-α, n = 5. I: In vitro sequestration of plasma LPS levels by cholestyramine (CHOL), polylysine cellulose resin (Poly-Lys), and polystyrene (PS). Each experiment was repeated three times. * or #, P < 0.05; ** or ##, P < 0.01; ***P < 0.001. * and **Comparisons were made against the HD group. For panels A–H, comparisons were also made between the HD-CHOL and HD-PS groups. # and ##Comparisons were made against the HD+CHOL group. For panel I, the in vitro endotoxin depleting experiment, the comparisons were made for sequestration efficacy within each type of resin. Data were mean ± SEM.
Emerging evidence showed plasma endotoxin is critically related to MetS and NASH, suggesting a potential causal relationship (4,16,17). Indeed, the plasma endotoxin levels were elevated approximately twice in the HD-fed mice as in the control mice (Fig. 1G). As expected, the plasma endotoxin levels were substantially reduced through oral administration of cationic polystyrene, whereas polystyrene beads were without effect. The elevated plasma endotoxin may further trigger systemic inflammation that can subsequently induce insulin resistance. Indeed, TNF-α levels in the plasma, being elevated under HD feeding, were declined through cationic polystyrene treatment (Fig. 1H). Finally, through an in vitro binding assay, we confirmed that cholestyramine could effectively deplete endotoxin (Fig. 1I).
NASH Features Are Ameliorated by Cholestyramine Treatment
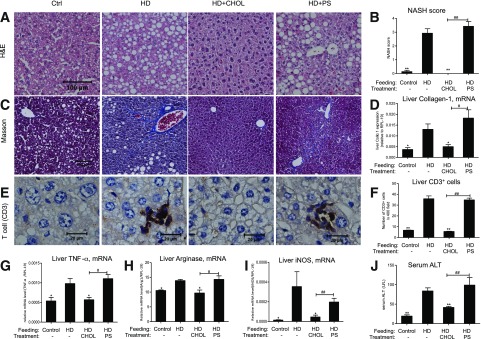
Hepatic steatosis and nonalcoholic fatty liver disease scores were evident in the parenchyma of the mice fed with HD (Fig. 2A and B). Moderate but evident fibrosis was present, as demonstrated by Masson’s trichrome staining (Fig. 2C) and the mRNA levels of liver collagen-α1 (Coll-α1) (Fig. 2D) in mice subjected to HD feeding for 22 weeks. Hepatic inflammation was further demonstrated by the presence of CD3+ T cells (Fig. 2E and F) and increased expression of inflammatory cytokines such as TNF-α, arginase 1, and inducible nitric oxide synthase (iNOS) (Fig. 2G–I). The remarkable elevation of serum alanine aminotransferase (ALT) (Fig. 2J) as an indicator of hepatic injury was also evident in the HD mice. Importantly, all of these NASH lesions were completely prevented by oral administration of cholestyramine, whereas polystyrene resin was without the effects. These results again imply the existence of negatively charged components in the gut that may impact the hepatocytes for steatosis and hepatic stellate cells for fibrosis in the HD-fed mice.
Figure 2.
NASH features are antagonized by cholestyramine treatment. The liver tissues and serum ALT levels of the mice described in Fig. 1 were assessed for NASH characteristics. A: Hematoxylin-eosin (H&E) staining. B: Hepatic steatosis and lesions were assessed for NASH based on H&E staining. C: Masson’s trichrome staining for liver fibrosis. D: Hepatic gene expression of Coll-α1, n = 6–7. E and F: Immunohistochemical staining of T-cell marker CD3 of liver tissue. CD3+ cell number counting on liver tissue stained with CD3 (×400-fold). Liver inflammation as measured by gene expression of TNF-α (G), arginase 1 (H), and iNOS (I), n = 4–6. J: Serum ALT levels, n = 8–10. *P < 0.05; **P < 0.01; ***P < 0.001. * and **Comparisons were made against the HD group. # and ##Comparisons were made against the HD+CHOL group. Data were mean ± SEM. Ctrl, control.
Small Intestinal Inflammation and Permeability Are Ameliorated by Cholestyramine
The pathological findings in the liver could be mediated through impaired enterohepatic circulation. As expected, the mice fed HD exhibited inflammation in the ileum, as indicated by the expression of two proinflammatory cytokines (Supplementary Fig. 1A and B). The inflammation-exerted intestinal lesion was also demonstrated by the increased gut permeability (Supplementary Fig. 1C). Furthermore, the increment of gut permeability was associated with decreased expression of tight-junction components, including ZO1, claudin 2, and occludin by HD-fed mice (Supplementary Fig. 1D). Expression of muc2, a major gene for intestinal mucus, was also downregulated, leading to impairment of mucus in the ileum (Supplementary Fig. 1E–G). Importantly, oral administration of cholestyramine, but not polystyrene, substantially antagonized the aforementioned intestinal lesions. Again, these results demonstrated that the negatively charged pathogenic components might cause intestinal injury and subsequent fatty liver.
Gut Dysbiosis Is Resolved by Cholestyramine Treatment
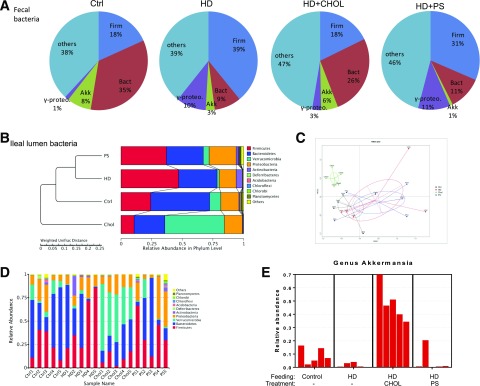
The elevation of plasma endotoxin by the HD feeding may come from gut dysbiosis and the impairment of intestinal interface. For the control chow feeding, Bacteroidetes at the phylum level was dominant over Firmicutes (Fig. 3A). In contrast, the abundance of Bacteroidetes was declined, whereas the abundance of Firmicutes was greatly increased in the mice fed with HD. The results were in agreement with the clinical findings, showing increased Firmicutes and decreased Bacteroidetes in obese patients (7,18). Importantly, cholestyramine treatment restored eubiosis, showing reduction of Firmicutes and restoration of Bacteroidetes. Restoration of Bacteroidetes, a major group of Gram-negative microbes that generate endotoxin at their death, is in agreement with the reduction of plasma endotoxin levels by cationic beads.
Figure 3.
Gut dysbiosis is rebalanced through cationic polystyrene treatment. The microbiota in the ileum and feces of the mice described in Fig. 1 was examined. A: The abundances of phyla Firmicutes (Firm) and Bacteroidetes (Bact), species A. muciniphila (Akk), and class Gammaproteobacteria (γ-proteo) in the feces were detected by 16S rDNA-qPCR (n = 6 for each group). B: The bacteria population in the lumen of ileum was determined by high-throughput sequencing analysis (n = 5 for each group). UPGMA (unweighted pair-group method with arithmetic mean) clustering based on weighted unifrac distance of the top 10 relative abundant phyla. C: NMDS (non-metric multidimensional scaling) plot; every point shows one mouse, different colors signify different groups, and the stress = 0.127. D: The relative abundance of bacteria at phylum levels in the ileal lumen. E: The relative abundance of A. muciniphila in the ileal content. Ctrl, control.
Symbiotic Akkermansia muciniphila, which accounts for ∼8% of fecal bacteria for the control chow-fed mice, was decreased to 3% by the HD feeding. Cholestyramine treatment restored the A. muciniphila to a ratio of 6% among the total bacteria. We also examined the microbiota within ileum in the mice under the treatments. We applied 16S rDNA high-throughput sequencing to examine the gut microbiota. Through hierarchical clustering (Fig. 3B and C), closely similar gut microbiota appeared grouped together in two states: gut microbiota from the mice treated with cholestyramine were closely related to those in the control feeding, and the microbiota from the mice treated with polystyrene were closely related to the ones from the HD feeding. Again, the increase in phylum Firmicutes by HD feeding was suppressed by cholestyramine treatment. The loss of Bacteroidetes and A. muciniphila by the mice fed with HD was restored with cationic polystyrene treatment (Fig. 3D and E).
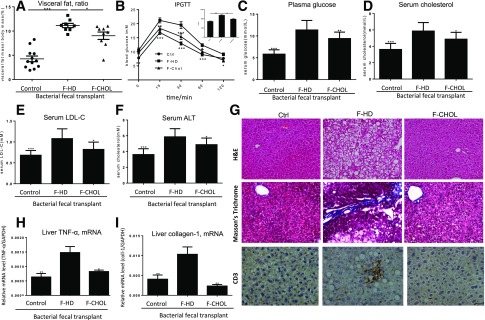
Microbes Derived From the Cholestyramine Treatment Are Sufficient to Antagonize the Metabolic Disorders of the Recipients
Given the fact of restoring of eubiosis and reduction of plasma endotoxin by cationic administration, we asked if the rebalanced gut microbiota was sufficient to antagonize the MetS. We performed FBT with two types of fecal microbes, one from cationic polystyrene and another from HD diet. Results showed that the gut microbiome derived from the cationic polystyrene-treated mice reduced body weight and visceral fat deposition of the recipients (Fig. 4A and Supplementary Fig. 2B). Likewise, IPGTT, fasting blood glucose, plasma cholesterol, and LDL were all suppressed, and the HLD-to-LDL ratio was restored to normal levels after FBT of the microbes derived from cationic polystyrene-treated animals (Fig. 4B–E). Liver injury (serum ALT), macrovesicular steatosis, and fibrosis of the recipients were relieved by FBT derived from cholestyramine treatment (Fig. 4F–I). Finally, our data showed that FBT could actually change the structure of the microbiota within the distal region of the small intestine of the recipients (Supplementary Fig. 3).
Figure 4.
FBT demonstrates that the microbes from the cholestyramine treatment are sufficient to antagonize the metabolic disorders of the recipient mice. The mice fed with HD or the control chow for 20 weeks were used as two types of recipients, which were subjected to gavage transplant from the donor microbes derived from cholestyramine-treated (F-CHOL) or HD (F-HD) mice (n = 10). A: Percent visceral fat deposition among the body mass, of the mice at the end of FBT treatment. B: IPGTT and area under the curve (AUC) of the mice at the end of FBT treatment. C: Fasting blood glucose of the mice at the end of FBT treatment. D: Plasma cholesterol. E: Plasma LDL-C. F: Serum ALT levels for liver injury. G: Hematoxylin-eosin (H&E) staining of liver tissues for hepatic steatosis; Masson’s trichrome staining of liver tissues for fibrosis; immunohistological staining of CD3+ lymphocytes for liver inflammation of the mice at the end of FBT. H: Liver inflammation was assessed by expression of TNF-α by the mice at the end of FBT. I: Liver fibrosis was assessed by expression of type I collagen. * or #, P < 0.05; ** or ##, P < 0.01. Comparisons were made against the F-HD group. Data were mean ± SEM.
Discussion
Epidemiological surveys and animal experiments show that high calories may not be sufficient to account for biogenesis of metabolic disorders, indicating that additional second hits are needed. Gut dysbiosis was overwhelming in the metabolic disorders, showing elevation of phylum Firmicutes and downregulation of phylum Bacteroidetes, the major Gram-negative bacteria. We thus reasoned that the reduced abundance of Bacteroidetes might be due to their death, which consequently elevates intestinal and plasma endotoxin, promoting insulin resistance and even NASH. In addition to its original designation as bile acid sequestrant to reduce plasma cholesterol, cholestyramine was found to promote GLP-1 expression through activation of TGR5, a G protein–coupled receptor for bile acids (19). A small-sized clinical study showed that colestimide could improve NASH, but the underlying mechanism is unknown (20). Another clinical study applied bile acid sequestrant to treat patients with type 2 diabetes (21). The authors found that the bile acid levels were not correlated with the efficacy of the treatment, which raises a question for the actual role of bile acid sequestration in the treatment. The mechanism for the cationic resin–mediated restoration of eubiosis is unknown. However, the critical role of the positive charges of the resin indicates that the negatively charged endotoxin along with other microbe products, such as DNA fragments, and short-chain fatty acids may be critical as well. Another interesting question that should be further addressed is how A. muciniphila, a major symbiotic species that has the power to antagonize metabolic disorders in the gut, is substantially recovered in the gut by cationic resin treatment.
Conclusion
We found that oral administration of cationic resin, such as cholestyramine, can thoroughly reduce plasma endotoxin, promotes the gut eubiosis, resolves inflammation, restores insulin responsiveness, and antagonizes hepatic steatosis and fibrosis. Moreover, FBT demonstrated that the rebalanced gut microbes from the cholestyramine-treated animal were sufficient to reverse the metabolic disorders, again showing critical roles of gut microbes in maintaining health. Our findings also provide preclinical justification for clinical trials for treatment of NASH and MetS through cationic polymers to sequestrate the dysbiosis that produced negatively charged components in the gut.
Supplementary Material
Article Information
Funding. This study was supported by the National Natural Science Foundation of China (31571165), Science and Technology Department of Sichuan Province, and the National Institute of Diabetes and Digestive and Kidney Diseases (P01-CA-163200-01 and P01-DK-098108 to S.J.P.).
Duality of Interest. Y.-P.H. has received financial assistance from Chengdu Tongde Pharmaceutical Co. Ltd. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.Z., J.C., P.W., M.L., H.Z., L.Z., Z.C., W.L., and D.S. conducted experiments and performed data analysis. Y.Z., Y. Liu, Q.S., Y.D., Z.X., Z.D., S.Z., L.B., X.Z., Z.J., Y. Li, and R.H. participated in the discussion. S.J.P. and Y.-P.H. conceived the project and wrote the manuscript. Y.-P.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at Digestive Disease Week, Chicago, IL, 6–9 May 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0070/-/DC1.
References
- 1.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am 2004;33:351–375 [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2010;2:180–193 [DOI] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cani PD, Amar J, Iglesias MA, et al. . Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 5.Le Chatelier E, Nielsen T, Qin J, et al.; MetaHIT consortium . Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–546 [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. . A core gut microbiome in obese and lean twins. Nature 2009;457:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 8.Bohinc BN, Diehl AM. Mechanisms of disease progression in NASH: new paradigms. Clin Liver Dis 2012;16:549–565 [DOI] [PubMed] [Google Scholar]
- 9.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836–1846 [DOI] [PubMed] [Google Scholar]
- 10.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–845 [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353–373 [DOI] [PubMed] [Google Scholar]
- 12.Barchetta I, Angelico F, Del Ben M, et al. . Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 2011;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han YP, Kong M, Zheng S, et al. . Vitamin D in liver diseases: from mechanisms to clinical trials. J Gastroenterol Hepatol 2013;28(Suppl. 1):49–55 [DOI] [PubMed] [Google Scholar]
- 14.Kong M, Zhu L, Bai L, et al. . Vitamin D deficiency promotes nonalcoholic steatohepatitis through impaired enterohepatic circulation in animal model. Am J Physiol Gastrointest Liver Physiol 2014;307:G883–G893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su D, Nie Y, Zhu A, et al. . Vitamin D signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol 2016;7:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J, Baker SS, Liu W, et al. . Endotoxemia unrequired in the pathogenesis of pediatric nonalcoholic steatohepatitis. J Gastroenterol Hepatol 2014;29:1292–1298 [DOI] [PubMed] [Google Scholar]
- 17.Wong VW, Wong GL, Chan HY, et al. . Bacterial endotoxin and non-alcoholic fatty liver disease in the general population: a prospective cohort study. Aliment Pharmacol Ther 2015;42:731–740 [DOI] [PubMed] [Google Scholar]
- 18.Lê KA, Li Y, Xu X, et al. . Alterations in fecal Lactobacillus and Bifidobacterium species in type 2 diabetic patients in Southern China population. Front Physiol 2013;3:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe M, Morimoto K, Houten SM, et al. . Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS One 2012;7:e38286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniai M, Hashimoto E, Tobari M, et al. . Treatment of nonalcoholic steatohepatitis with colestimide. Hepatol Res 2009;39:685–693 [DOI] [PubMed] [Google Scholar]
- 21.Brufau G, Bahr MJ, Staels B, et al. . Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab (Lond) 2010;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.