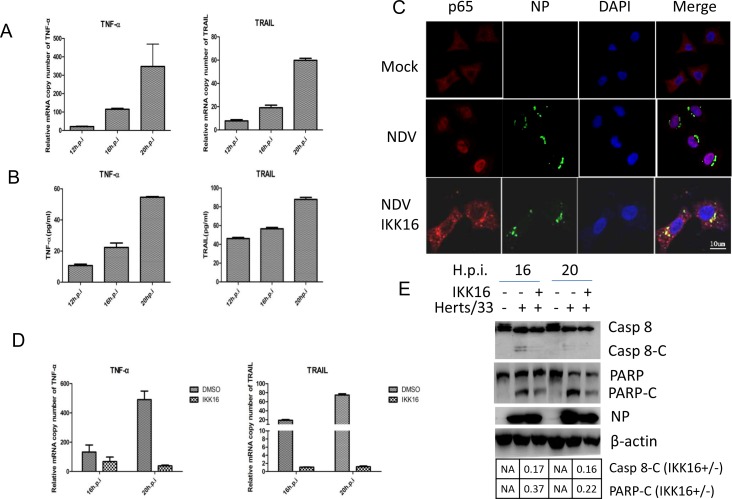
Figure 2. TNF-α and TRAIL are up-regulated via NF-кB pathway and promotes caspase 8 activation in NDV-infected HeLa cells.
(A) Transcriptional up-regulation of TNF-α and TRAIL during NDV infection. HeLa cells were mock-infected or NDV-infected for 12, 16, or 20 h. Total RNA was extracted and the expression levels of TNF-α and TRAIL were analyzed with semi-quantitative real time RT-PCR. The values represent means of three independent triplicate experiments ± S.D. (B) Secretion of TNF-α and TRAIL. The culture medium was collected at 12, 16, 20 h.p.i., and subjected to ELISA with anti-TNF-α or anti-TRAIL. The values represent means of three independent triplicate experiments ± S.D. (C) The nuclear translocation of p65 during NDV infection and blockage of p65 nuclear translocation by IKKβ inhibitor IKK16. HeLa cells were mock–infected or NDV-infected, followed by the treatment of DMSO or 5 μM IKK16. The translocation of p65 (red) and the expression of viral protein NP (green) were analyzed with immunostaining at 16 h.p.i. using anti-p65 and anti-NP. Nuclei were stained with DAPI (blue). Images were acquired by a META 510 confocal laser-scanning microscope (Zeiss). Merged images illustrate DAPI/NP/p65 fluorescence. (D) The inhibition of TNF-α and TRAIL transcription by IKK16. HeLa cells were mock-infected or NDV-infected, followed by the treatment of DMSO or 5 μM IKK16. Total RNA was extracted 16 and 20 h.p.i., and subjected to semi-quantitative real time RT-PCR using primers targeting to TNF-α and TRAIL mRNA, respectively. The values represent means of three independent triplicate experiments ± S.D. (E) Inhibition of apoptosis by IKK16. Cell samples in Figure 1D were lysed and analyzed by Western blot using anti-caspase 8 and anti-PARP. The intensities of bands were determined by densitometry, normalized to β-actin, and shown as fold change (IKK16+/−).