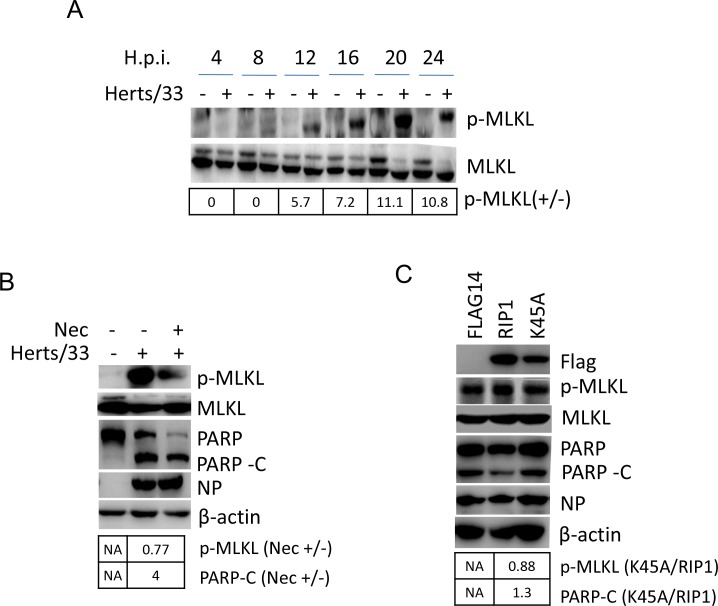
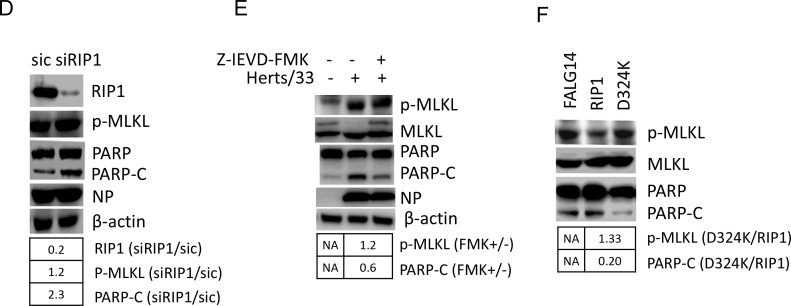
Figure 6. RIP1 regulates apoptosis and necroptosis during NDV infection.
(A) Phosphorylation of MLKL at Ser358. HeLa cells were mock-infected or NDV-infected. Cells were harvested at 4, 8, 12, 16, 20, and 24 h.p.i., and the phosphorylation level of MLKL at Ser358 was examined with Western blot using anti-phosphor-MLKL (Ser358). The intensities of phosphor-MLKL bands were determined by densitometry, normalized to MLKL, and shown as fold change (NDV : mock). (B) Inhibition of RIP1 kinase activity reduces necroptosis and promotes apoptosis. HeLa cells were infected with NDV, followed by treatment of DMSO or 200 μM RIP1 inhibitor Necrostain. Cells were harvested at 20 h.p.i., and the phosphorylation level of MLKL and cleavage of PARP were examined with Western blot. The intensities of bands were determined by densitometry. Phosphor-MLKL was normalized to MLKL, PARP-C was normalized to β-actin, and were shown as fold change (Nec +/−). (C) RIP1 kinase activity is anti-apoptotic. HeLa cells were transfected with Flag14, RIP1, and K45A for 20 h, followed by NDV infection. Cells were harvested at 20 h.p.i., and analyzed with Western blot. The intensities of bands were determined by densitometry. Phosphor-MLKL was normalized to MLKL, PARP-C was normalized to β-actin, and were shown as fold change (K45A/RIP1). (D) Depletion of RIP1 promotes apoptosis. HeLa cells were transfected with siRNA targeting to RIP1 or control siRNA (sic) for 36 h, followed by NDV infection. Cells were harvested at 20 h.p.i., and analyzed with Western blot. The intensities of bands were determined by densitometry. Phosphor-MLKL was normalized to MLKL, RIP1 and PARP-C were normalized to β-actin, and were shown as fold change (siRIP1/sic). (E) Inhibition of caspase 8 activity and RIP1 cleavage promotes necroptosis and reduces apoptosis. HeLa cells were mock-infected or NDV-infected, followed by treatment of DMSO or 40 μM caspase 8 inhibitor Z-IEVD-FMK. Cells were harvested at 20 h.p.i., and the phosphorylation level of MLKL and cleavage of PARP was examined by Western blot. The intensities of bands were determined by densitometry. Phosphor-MLKL was normalized to MLKL, PARP-C was normalized to β-actin, and were shown as fold change (FMK +/−). (F) Blockage of RIP1 cleavage by mutating D324 to K promotes necroptosis and reduces apoptosis during NDV infection. HeLa cells were transfected with Flag14, RIP1, and D324K for 20 h, followed by NDV infection. Cells were harvested at 20 h.p.i., and analyzed with Western blot. The intensities of bands were determined by densitometry. Phosphor-MLKL was normalized to MLKL, PARP-C was normalized to β-actin in Figure 4C, and were shown as fold change (D324K/RIP1).