Abstract
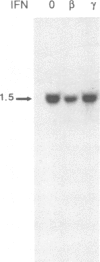
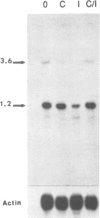
As an approach to identifying genes involved in physiological actions of interferons we used differential probes to screen a cDNA library from mouse L-929 cells treated with interferon alpha/beta. We identified two negatively regulated mRNA species which have been examined by analysis of the corresponding mRNAs and by DNA sequencing. Comparison with the GenBank database showed that these cDNA clones corresponded to mitochondrially encoded genes for cytochrome b and subunit I of cytochrome c oxidase. A further cDNA encompassing three mitochondrial genes was used as a probe to show that a third mRNA, NADH dehydrogenase subunit 5, was also down-regulated by interferon while a fourth, NADH dehydrogenase subunit 6, was unaffected. Expression of cytochrome b was also inhibited in mouse NIH 3T3 cells treated with interferon alpha/beta and in human Daudi lymphoblastoid cells treated with interferon alpha. The ability of interferon to reduce mitochondrial mRNA levels could be blocked by cycloheximide suggesting that these effects are mediated by an interferon-responsive nuclear gene which encodes a product capable of regulating mitochondrial gene expression. Analysis of proteins synthesized in the presence of emetine, a specific inhibitor of cytoplasmic translation, showed that the synthesis of several mitochondrial translation products, including cytochrome b, was reduced after treatment with interferon. Our results reveal a novel effect of interferon on cellular physiology which could have important consequences for understanding the effects of interferons as well as suggesting new mechanisms for the regulation of mitochondrial biogenesis and function.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987 Jul 31;50(3):331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Haller O. Antiviral state against influenza virus neutralized by microinjection of antibodies to interferon-induced Mx proteins. EMBO J. 1988 May;7(5):1315–1320. doi: 10.1002/j.1460-2075.1988.tb02946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Taylor-Papadimitriou J. Interferon affects both G1 and S+G2 in cells stimulated from quiescence to growth. Nature. 1978 Aug 24;274(5673):798–800. doi: 10.1038/274798a0. [DOI] [PubMed] [Google Scholar]
- Bhat K. S., Bhat N. K., Kulkarni G. R., Iyengar A., Avadhani N. G. Expression of the cytochrome b-URF6-URF5 region of the mouse mitochondrial genome. Biochemistry. 1985 Oct 8;24(21):5818–5825. doi: 10.1021/bi00342a020. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A mammalian mitochondrial RNA processing activity contains nucleus-encoded RNA. Science. 1987 Mar 6;235(4793):1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebath J., Benech P., Hovanessian A., Galabru J., Revel M. Four different forms of interferon-induced 2',5'-oligo(A) synthetase identified by immunoblotting in human cells. J Biol Chem. 1987 Mar 15;262(8):3852–3857. [PubMed] [Google Scholar]
- Chebath J., Benech P., Revel M., Vigneron M. Constitutive expression of (2'-5') oligo A synthetase confers resistance to picornavirus infection. Nature. 1987 Dec 10;330(6148):587–588. doi: 10.1038/330587a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Product of Saccharomyces cerevisiae nuclear gene PET494 activates translation of a specific mitochondrial mRNA. Mol Cell Biol. 1986 Nov;6(11):3694–3703. doi: 10.1128/mcb.6.11.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Koerner T. J., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP1, a yeast nuclear gene involved in 5' end processing of cytochrome b pre-mRNA. J Biol Chem. 1984 Apr 25;259(8):4722–4731. [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Inhibitory effects of interferon on the expression of genes regulated by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7608–7612. doi: 10.1073/pnas.82.22.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M. Analysis of replicating vaccinia DNA in interferon-treated, virus-infected cells. J Interferon Res. 1984 Spring;4(2):179–192. doi: 10.1089/jir.1984.4.179. [DOI] [PubMed] [Google Scholar]
- Faye G., Simon M. Analysis of a yeast nuclear gene involved in the maturation of mitochondrial pre-messenger RNA of the cytochrome oxidase subunit I. Cell. 1983 Jan;32(1):77–87. doi: 10.1016/0092-8674(83)90498-1. [DOI] [PubMed] [Google Scholar]
- Feng G. S., Taylor M. W. Interferon gamma-resistant mutants are defective in the induction of indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7144–7148. doi: 10.1073/pnas.86.18.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Vogel S. N. Interferons with special emphasis on the immune system. Adv Immunol. 1983;34:97–140. doi: 10.1016/s0065-2776(08)60378-8. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Moore G., Tilleray V. J., Clemens M. J. Inhibition of cell proliferation by interferons. 1. Effects on cell division and DNA synthesis in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1984 Mar 15;139(3):619–625. doi: 10.1111/j.1432-1033.1984.tb08049.x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak G. J., Knight E., Jr Selective reduction of c-myc mRNA in Daudi cells by human beta interferon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1747–1750. doi: 10.1073/pnas.81.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Gilbert C. S., Stark G. R., Kerr I. M. Differential regulation of interferon-induced mRNAs and c-myc mRNA by alpha- and gamma-interferons. Eur J Biochem. 1985 Dec 2;153(2):367–371. doi: 10.1111/j.1432-1033.1985.tb09312.x. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kortsaris A., Taylor-Papadimitriou J., Georgatsos J. G. Interferon inhibition of protein synthesis by isolated mitochondria. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1317–1322. doi: 10.1016/0006-291x(76)90340-5. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Clive D. R., Zarlenga D. S., Greene J. J. Identification of interferon-modulated proliferation-related cDNA sequences. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8453–8457. doi: 10.1073/pnas.84.23.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse M., Dujardin G., Slonimski P. P. The yeast nuclear gene NAM2 is essential for mitochondrial DNA integrity and can cure a mitochondrial RNA-maturase deficiency. Cell. 1985 May;41(1):133–143. doi: 10.1016/0092-8674(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Esteban M. Induction of an anti-viral response and 2',5'-oligo A synthetase by interferon in several thymidine kinase-deficient cell lines. Virology. 1984 Mar;133(2):464–469. doi: 10.1016/0042-6822(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lewis J. A. Induction of an antiviral state by interferon in the absence of elevated levels of 2,5-oligo(A) synthetase and eIF-2 kinase. Virology. 1988 Jan;162(1):118–127. doi: 10.1016/0042-6822(88)90400-x. [DOI] [PubMed] [Google Scholar]
- Lin S. L., Ts'o P. O., Hollenberg M. D. The effects of interferon on epidermal growth factor action. Biochem Biophys Res Commun. 1980 Sep 16;96(1):168–174. doi: 10.1016/0006-291x(80)91196-1. [DOI] [PubMed] [Google Scholar]
- McGraw P., Tzagoloff A. Assembly of the mitochondrial membrane system. Characterization of a yeast nuclear gene involved in the processing of the cytochrome b pre-mRNA. J Biol Chem. 1983 Aug 10;258(15):9459–9468. [PubMed] [Google Scholar]
- Moore G., Gewert D. R., Clemens M. J. Inhibition of cell proliferation by interferons. 2. Changes in processing and stability of newly synthesized DNA in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1984 Mar 15;139(3):627–635. doi: 10.1111/j.1432-1033.1984.tb08050.x. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Edelstein M. P., Duch D. S. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1242–1246. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M. F., Kimchi A. Initial characterization of a spontaneous interferon secreted during growth and differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1982 Dec;2(12):1472–1480. doi: 10.1128/mcb.2.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Duncan R., Hershey J. W., Kerr I. M. Double-stranded RNA-dependent protein kinase and 2-5A system are both activated in interferon-treated, encephalomyocarditis virus-infected HeLa cells. J Virol. 1985 Jun;54(3):894–898. doi: 10.1128/jvi.54.3.894-898.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtsmeier W. J., Grossberg S. E. Inhibitory effects of mitochondrial metabolic inhibitors on interferon action. J Interferon Res. 1989 Feb;9(1):87–96. doi: 10.1089/jir.1989.9.87. [DOI] [PubMed] [Google Scholar]
- Rödel G., Fox T. D. The yeast nuclear gene CBS1 is required for translation of mitochondrial mRNAs bearing the cob 5' untranslated leader. Mol Gen Genet. 1987 Jan;206(1):45–50. doi: 10.1007/BF00326534. [DOI] [PubMed] [Google Scholar]
- Samid D., Chang E. H., Friedman R. M. Biochemical correlates of phenotypic reversion in interferon-treated mouse cells transformed by a human oncogene. Biochem Biophys Res Commun. 1984 Feb 29;119(1):21–28. doi: 10.1016/0006-291x(84)91612-7. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Söllner T., Schweyen R. J. Nuclear suppression of a mitochondrial RNA splice defect: nucleotide sequence and disruption of the MRS3 gene. Mol Gen Genet. 1987 Nov;210(1):145–152. doi: 10.1007/BF00337771. [DOI] [PubMed] [Google Scholar]
- Shan B., Lewis J. A. Interferon-induced expression of different genes is mediated by distinct regulatory pathways. Virology. 1989 May;170(1):277–281. doi: 10.1016/0042-6822(89)90378-4. [DOI] [PubMed] [Google Scholar]
- Sharp N. A., Luscombe M. J., Clemens M. J. Regulation of c-fgr proto-oncogene expression in Burkitt's lymphoma cells: effect of interferon treatment and relationship to EBV status and c-myc mRNA levels. Oncogene. 1989 Aug;4(8):1043–1046. [PubMed] [Google Scholar]
- Sokawa Y., Watanabe Y., Watanabe Y., Kawade Y. Interferon suppresses the transition of quiescent 3T3 cells to a growing state. Nature. 1977 Jul 21;268(5617):236–238. doi: 10.1038/268236a0. [DOI] [PubMed] [Google Scholar]
- Soslau G., Bogucki A. R., Gillespie D., Hubbell H. R. Phosphoproteins altered by antiproliferative doses of human interferon- beta in a human bladder carcinoma cell line. Biochem Biophys Res Commun. 1984 Mar 30;119(3):941–948. doi: 10.1016/0006-291x(84)90864-7. [DOI] [PubMed] [Google Scholar]
- St Laurent G., Yoshie O., Floyd-Smith G., Samanta H., Sehgal P. B., Lengyel P. Interferon action: two (2'-5')(A)n synthetases specified by distinct mRNAs in Ehrlich ascites tumor cells treated with interferon. Cell. 1983 May;33(1):95–102. doi: 10.1016/0092-8674(83)90338-0. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Takikawa O., Kuroiwa T., Yamazaki F., Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988 Feb 5;263(4):2041–2048. [PubMed] [Google Scholar]
- Tominaga S., Lengyel P. beta-Interferon alters the pattern of proteins secreted from quiescent and platelet-derived growth factor-treated BALB/c-3T3 cells. J Biol Chem. 1985 Feb 25;260(4):1975–1978. [PubMed] [Google Scholar]
- Wells V., Mallucci L. Expression of the 2-5A system during the cell cycle. Exp Cell Res. 1985 Jul;159(1):27–36. doi: 10.1016/s0014-4827(85)80034-3. [DOI] [PubMed] [Google Scholar]