Figure 2. Appearance of limited degree of immunogenicity in L5178Y DBA/2 leukemia cells exposed to early transplant generations of DTIC treatment, revealed by immuno-chemotherapy synergism.
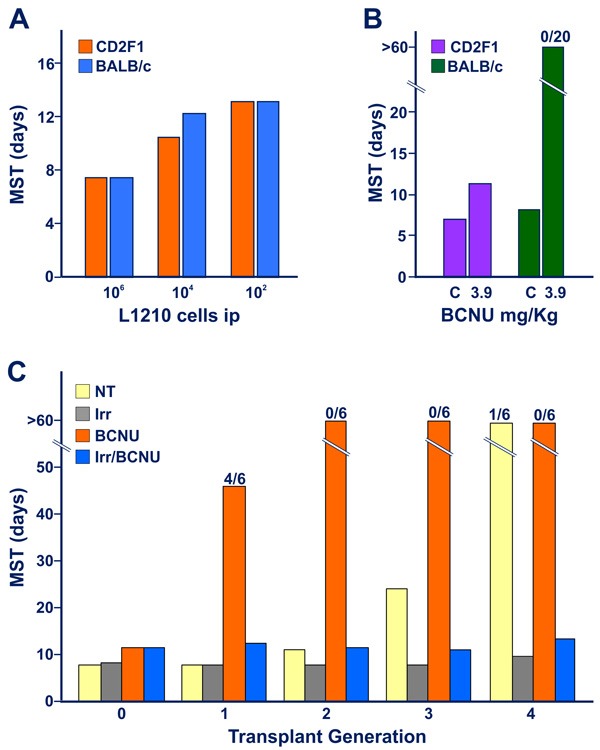
When not specified in terms of number of dead mice over the total tested indicated on the top of the columns, all mice (6-8 animals for group) died with generalized leukemia. Figure 2A. Graded numbers of L1210 leukemia cells were inoculated into fully histocompatible CD2F1 mice, or into H-2d-compatible BALB/c mice, incompatible for multiple minor histocompatibility antigens (see Ref 17). The marginal allograft response of BALB/c hosts was not adequate to restrain the growth of leukemic cells, as evidenced by the finding that no substantial difference in median survival time was detected between CD2F1 and BALB/c mice inoculated with as low as 102 L1210 cells ip. Figure 2B. CD2F1 and BALB/c mice were inoculated with 105 L1210 cells ip. In this case, CD2F1 mice treated with a low dose of BCNU, (3.9 mg/Kg ip, administered on day 3 after tumor transplantation) showed a limited increase in MST with respect to that of untreated controls (C). In contrast, all BCNU-treated allogeneic BALB/c mice, survived beyond the 60 day-observation period, thus confirming the possibility of revealing a marginal antitumor graft response of the host through an immune-chemotherapy synergistic effect. Figure 2C. (data from Ref 13). The strategy of immune-chemotherapy synergism indicates that murine leukemia cells exposed in vivo to DTIC acquire appreciable levels of immunogenicity already at transplant generation “1”. Malignant cell immunogenicity progressively increases at the successive generations of treatment with the triazene compound. This figure illustrates the results of a typical experiment performed to evaluate the immunogenic properties of L5178Y leukemia cells in the course of the first 4 transplant generations of DTIC treatment (DTIC 100 mg/Kg/day ip for 10 days) in CD2F1 mice. Blasts (105 cells) obtained from non-treated leukemic donors (i.e. at transplant generation “0”) or from DTIC-treated leukemic donors (at transplant generations 1 through 4) were inoculated into 4 groups of mice, i.e. non-treated (NT), immunodepressed through exposure to total-body irradiation (Irr, 4 Gy X rays, on day -1), treated with BCNU (10 mg/Kg ip), immunodepressed (i.e. pre-irradiated) and treated with BCNU (Irr/BCNU). An additional group of mice was treated with DTIC to obtain a further generation of treatment with the triazene compound. At transplant generation “0” the intact L5178Y cells did not show appreciable immunogenicity, since all non-immunodepressed or irradiated recipients treated with BCNU showed a similar modest increase of MST over that of non-treated controls. Remarkably, at transplant generation “1” instead, L5178Y cells obtained from DTIC-treated donors showed immunogenicity strength similar to that conferred by products of minor histocompatibility loci. This is evidenced by the consistent increase of survival times of BCNU-treated animals respect to those of mice not subjected to chemotherapy, or treated with BCNU but immunodepressed by means of total-body irradiation.
