Figure 1. Formation of S-S oligomers in SOD1-related ALS.
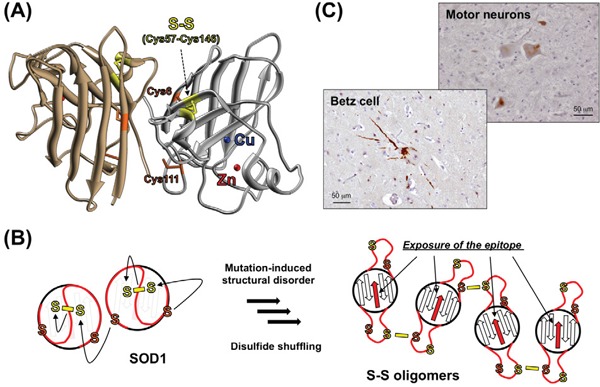
A. Crystal structure of a natively folded SOD1 with copper (blue) and zinc (red) ions and an intramolecular disulfide bond between Cys57 and Cys146 (yellow) (PDB ID: 1HLA). Cys6 and Cys111 are also shown (orange). B. A disulfide-shuffling model for the formation of S-S oligomers. Cys6/111 (orange) attack Cys57/146 (yellow) forming the disulfide bond, which shuffles the disulfide bond among four Cys residues to form S-S oligomers. Upon formation of the S-S oligomers, the epitope for our anti-oligomer antibody (red arrows), which is buried in the folded conformation, becomes exposed and available for immunohistochemical examination. C. Immunohistochemical examination on Betz cells and spinal motor neurons in the ALS patients with SOD1 mutation (C111Y) using anti-oligomer antibody. Nuclei were also stained by hematoxylin (blue).
