Abstract
Glasgow prognostic score (GPS) has been reported to be an indicator of prognosis for various cancers. However, the relationship between GPS and colorectal cancers (CRC) remains unclear. A comprehensive search of Pubmed, Embase, Cochrane library, Web of Science, ChinaInfo and Chinese National Knowledge Infrastructure was performed to identify eligible studies, from which the risk of overall survival (OS) and cancer-specific survival (CSS) were extracted. A random-effect model was adopted to combine hazard ratio (HR) and 95% confidence interval (CI). 25 articles with a total of 5660 participants were included. The pooled results indicated that elevated GPS was associated with poor OS (HR = 2.83, 95%CI: 2.00–4.00, P < 0.01) and CSS (HR = 1.94, 95%CI: 1.51–2.49, P < 0.01). This correlation was confirmed both in primary operable and advanced inoperable patients. Increased GPS was also closely related to advanced tumour-node-metastasis (TNM) stage (odds ratio [OR] = 1.44, 95% CI: 1.010–2.065, P < 0.05) and elevated level of serum carcinoembryonic antigen (OR = 2.252, 95% CI: 1.508–3.362, P < 0.01). Subgroup analysis revealed a significant association between high GPS and poor survival outcome according to the factors of sample size, study of region and cut-off value of GPS level. These findings suggest that GPS may serve as a reliable predictive index for patients with CRC.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and accounts for 10% of all newly diagnosed cancers1. Although the surgical techniques, chemotherapy and molecular-target therapy have dramatically developed, the long-term survival rate of patients with CRC remains low, particularly in patients with advanced stage cancers2. Accurate prediction of prognosis will assist in adopting appropriate therapies and contribute to better management of CRC patients. Currently, the tumour-node-matastasis (TNM) surgical staging system, based on postoperative histopathology of the tumour, is considered to be the golden standard for predicting clinical outcomes of cancer patients3. However, its accuracy has been debated as tumour progression may not be solely determined by the characteristics of the tumour, but the host inflammatory responses as well4–6. Therefore, in recent years, great efforts have been made to identify inflammation-related factors for precise prediction of disease prognosis.
Glasgow Prognostic Score (GPS) is such an inflammation-based factor, defined by the combination of the level of serum C-reactive protein (CRP) and albumin, which are indicators of systematic inflammatory response and nutritional status respectively7. The first study on GPS reported by Forrest et al.8 in 2003 showed that it could be an predictor of prognosis for non-small-cell lung cancer patients. Subsequently, growing evidence suggested that GPS was served as an independent prognostic index in a variety of malignant cancers including hepatocellular carcinoma9, esophageal cancer10, gastric cancer11, renal cancer12, and pancreatic cancer13. For patients with CRC, the GPS system was also widely studied, but the results were controversial. Leitch and his colleagues14 reported that GPS was an independent prognostic factor for CRC, while Son et al.15 did not detect significant association between GPS and patients’ survival. This makes it rational to perform systematic evaluation of the correlation between GPS and prognosis of patients with CRC to further clarify its clinical significance.
Results
Characteristics of the enrolled studies
As shown in Fig. 1, a total of 710 records were identified from different databases by initial search. After screening the titles and abstracts, 73 full-text articles were assessed for further eligibility analysis. 48 of them were subsequently excluded as follows: 18 studies without sufficient data; 22 of overlapping populations; 8 of only documented with abstracts, comments or reviews. Finally, 25 original reports published between the year 2006 and 2016 with sample size ranging from 42 to 1000 were enrolled into the meta-analysis14–38. The characteristics of all the studies were summarized in Table 1.
Figure 1.
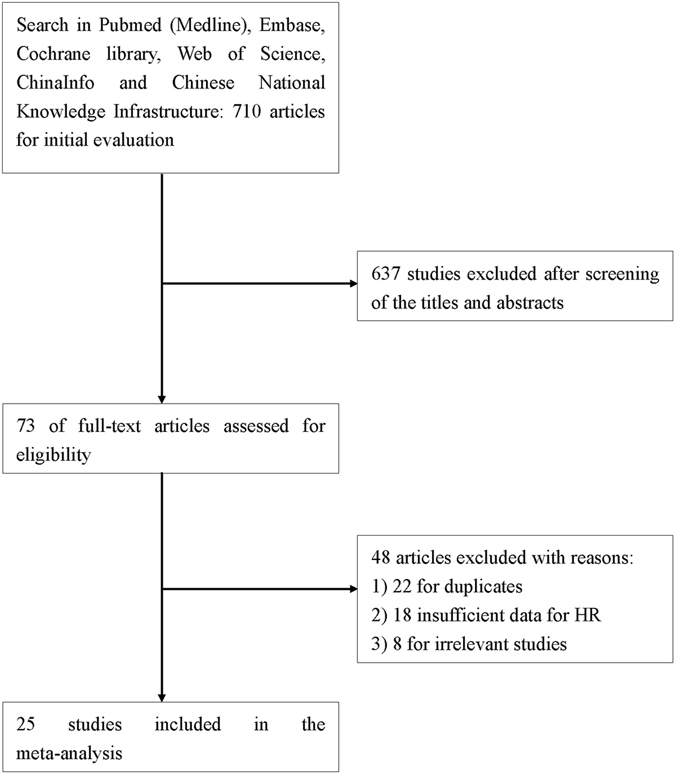
Flow diagram of the study selection process.
Table 1.
Characteristics of included studies.
| First Author | Year | Study region | Number (M/F) | Age (years) | Treatment | Follow-up | Stage |
|---|---|---|---|---|---|---|---|
| Read16 | 2006 | Australia | 51 (30/21) | 64 (40–79)a | chemotherapy | Median: 29.8 months | IV |
| Ishizuka17 | 2007 | Japan | 315 (186/129) | <70: 162; ≥70: 153 | surgery | 11 days-13.7 months | 0-IV |
| Xiao22 | 2013 | China | 223 (148/75) | <65: 181; ≥65: 42 | Surgery | 43 (6–84) months | I-IV |
| Kobayashi24 | 2014 | Japan | 106 (77/29) | <70: 66; ≥70: 40 | Surgery | Median: 38 m | IV |
| Nozoe25 | 2014 | Japan | 272 (160/112) | 70.4 (24–90)a | Surgery | NR | I–IV |
| Lin26 | 2015 | China | 99 (35/64) | 62.63 ± 10.86b | Surgery | Median: 60 months | II |
| Shibutani27 | 2015 | Japan | 254 (139/115) | 60 (26–86)a | surgery | NR | II、III |
| Ishizuka28 | 2016 | Japan | 627 (400/227) | 67.75 ± 11.69b | Surgery | NR | 0–IV |
| Eren29 | 2016 | Turkey | 115 (64/51) | 66.1 ± 12.77b | Surgery | 20 (7–41) months | I–IV |
| Kishiki32 | 2013 | Japan | 79 (42/37) | ≤70: 43; >70: 36 | Surgery | 32 (1–66) months | IV |
| Son15 | 2013 | Korea | 624 (368/256) | <60: 295; ≥60: 329 | Surgery | 42 (1–66) months | I–III |
| Adachi35 | 2015 | Japan | 65 (37/28) | 64 (17–83)a | surgery | NR | I–IV |
| Ghanim36 | 2015 | Austria | 52 (31/21) | 62.7 ± 11.4b | surgery | NR | IV |
| Song37 | 2015 | Koea | 177 (83/94) | 52 (25–81)a | Korean treatmen | 3.1 (0.1–33.3) months | IV |
| Toiyama30 | 2011 | Japan | 219 (136/83) | 66 (58–73)a | surgery | 52.7 (56.9 63.8) months | II, III |
| Park38 | 2016 | UK | 1000 (548/452) | <65: 330; 65–74: 347; >75: 323 | surgery | 56 (10–206) months | 0–III |
| Choi23 | 2014 | Korea | 105 (63/42) | 63 (32–86)a | Surgery | 44 (2–81) months | I–IV |
| Leitch14 | 2007 | UK | 233 (129/104) | <65: 34; 65–74: 27; >75: 23 | Surgery + chemotherapy | 12 (6–73) months | I–IV |
| Manabu31 | 2012 | Japan | 42 (26/16) | <70: 12; ≥70: 30 | chemotherapy | Median: 424 days | IV |
| Inoue33 | 2013 | Japan | 245 (146/99) | 64 (29–85)a | chemotherapy | NR | IV |
| Nakagawa34 | 2014 | Japan | 343 (219/124) | 62.83 ± 3.85 | surgery | NR | IV |
| Kobayashi18 | 2010 | Japan | 63 (44/19) | <70: 41; ≥70: 22 | surgery | 38 (30.5–45.6) months | I–IV |
| Furukawa19 | 2012 | Japan | 40 (30/10) | 66.1 ± 9.7b | chemotherapy | NR | IV |
| Sugimoto20 | 2012 | Japan | 366 (209/157) | ≤70: 240; >70: 126 | surgery | Median: 70.8 months | II, III |
| Madea21 | 2013 | Japan | 94 (51/43) | <70: 62; ≥70: 32 | surgery | Median: 21 months | IV |
| First Author | Survival analysis | Cut-off value | Lymphatic invasion (+/−) | Nevous invasion (+/−) | CEA (ng/ml) | Differentiation (well/moderate/poor) | |
| Read16 | OS | 1 | NR | NR | NR | NR | |
| Ishizuka17 | OS | 1 | NR | NR | <6: 185; ≥6: 120 | NR | |
| Xiao22 | OS | 1 | NR | NR | <5: 127; ≥5: 96 | NR | |
| Kobayashi24 | OS | 1 | 75/31 | 96/10 | <30: 75; ≥30: 56 | Well: 68; moderate & poor: 38 | |
| Nozoe25 | OS | 2 | 112/160 | 65/207 | NR | 82/170/20 | |
| Lin26 | OS | 2 | NR | 27/72 | ≤10: 73; >10: 26 | 25/50/24 | |
| Shibutani27 | OS | 1 | 184/47 | 68/170 | ≤5: 154; >5: 44 | Well & moderate: 234; poor & mucinous: 19 | |
| Ishizuka28 | OS | 2 | 129/498 | 131/496 | ≤8.7: 433; >8.7: 194 | Well or moderate: 583; others: 44 | |
| Eren29 | OS | 2 | 68/47 | NR | <5: 97; ≥5: 18 | 13/81/11 | |
| Kishiki32 | OS | 2 | NR | NR | <6: 19; ≥6: 60 | Well or moderate: 77; others: 2 | |
| Son15 | OS | 2 | NR | NR | <5: 450; ≥5: 172 | Low: 562; high: 62 | |
| Adachi35 | OS | 2 | NR | NR | <10: 25; ≥10: 40 | Well & moderate: 45; poor & undifferentiated: 14; unknown: 6 | |
| Ghanim36 | OS | 1 | NR | NR | NR | NR | |
| Song37 | OS | 1 | NR | NR | ≤5: 31; >5: 140 | NR | |
| Toiyama30 | OS、CSS | 1 | 191/28 | 96/123 | ≤6: 134; >6: 85 | Differentiated: 200; non-differentiated: 19 | |
| Park38 | OS、CSS | 1 | NR | 507/493 | NR | Well & moderate: 894; poor: 96 | |
| Choi23 | CSS | 2 | NR | NR | <5: 63; ≥5: 41 | Well & moderate: 59; poor: 42 | |
| Leitch14 | CSS | 1 | NR | NR | NR | NR | |
| Manabu31 | CSS | 2 | NR | NR | NR | NR | |
| Inoue33 | CSS | 2 | NR | NR | NR | Differentiated: 219; non-differentiated: 26 | |
| Nakagawa34 | CSS | 1 | NR | NR | 124.4 ± 116.6 | Moderate: 203; others: 128 | |
| Kobayashi18 | CSS | 1 | 49/14 | 53/10 | <30: 43; ≥30: 18 | Well: 51; moderate & poor: 12 | |
| Furukawa19 | CSS | 2 | NR | NR | <100: 22; ≥100: 18 | NR | |
| Sugimoto20 | CSS | 2 | 362/4 | 343/23 | ≤3: 154; >3: 212 | Well: 162; others: 204 | |
| Madea21 | CSS | 2 | NR | NR | NR | Wel & moderate: 79; others: 15 | |
NR = not reported, OS = overall survival, CSS = cancer-specific survival.
aMean (range).
bMean ± SD.
Among the included studies, some patients were enrolled multiple times for different purposes in publications. In Leitch and his colleagues’ research14, the patients with TNM stage I,IIand III were overlapped with those in Park’s study38. So we only extracted the data of patients with stage IV from the prior study. Among all the studies, 15 were conducted in Japan, 2 in the United Kingdom, 2 in China, 3 in Korea, 1 in Australia, 1 in Turkey and 1 in Austria. Ten studies only included patients with stage IV, one only included stage II and the remaining included a mixed population. The cut-off values of GPS were diverse. In 12 studies, GPS of 1 was defined as elevation, while in the remaining 13 literatures, GPS of 2 was considered as an increase. Surgery was the main treatment approach in 20 of the 25 included studies. A ‘Korean treatment’ was discussed in one study conducted in Korea, and the remaining four studies focused on chemotherapy.
GPS and survival outcome
Obvious heterogeneity was detected in this meta-analysis (OS: I 2 = 74.3%, P < 0.001; CSS: I 2 = 63.3%, P = 0.002), so the random-effect model was adopted to calculate the pooled HR and 95% CI values. There were 16 studies reporting the relationship between pretreatment GPS and OS in CRC patients. The pooled HR of 2.83 (95% CI: 2.0–4.0, P < 0.001) implied that CRC patients with elevated GPS were expected to have poor OS (Fig. 2A). There were 11 studies presenting the relationship between GPS and CSS. The pooled result showed significant association between elevated GPS and short CSS (HR = 1.94, 95% CI: 1.51–2.49, P < 0.001) (Fig. 2B). Furthermore, subgroup analysis was conducted, stratified by sample size, study of region and cut-off value of GPS. As shown in Table 2, increased GPS was also predicted to have a significant poor prognostic effect on survival in CRC patients in those subgroups.
Figure 2.
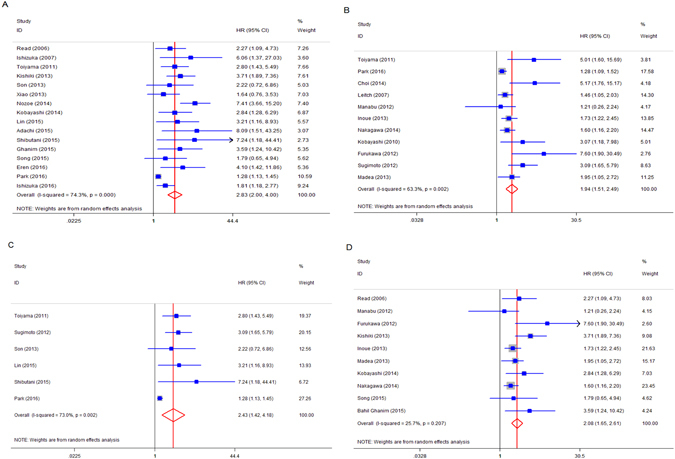
(A) Forest plot of hazard ratio for the association between GPS and OS in patients with CRC; (B) Forest plot of hazard ratio for the association betweenGPS and CSS; (C) Forest plot of hazard ratio for the association between elevated GPS and survival in patients with primary operable disease; (D) Forest plot of hazard ratio for the association between elevated GPS and survival in patients with advanced inoperable diseases.
Table 2.
Results of subgroup analysis.
| Subgroup | No. of studies | HR (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I 2 | Ph | ||||
| Overall survival | |||||
| Sample size | |||||
| >500 | 3 | 1.450 (1.100–1.913) | 0.008 | 36.3% | 0.208 |
| ≤500 | 13 | 3.284 (2.510–4.297) | <0.001 | 12.1% | 0.323 |
| Study of region | |||||
| Japan | 8 | 3.628 (2.344–5.615) | <0.001 | 53.7% | 0.034 |
| others | 8 | 2.038 (1.402–2.963) | <0.001 | 50.3% | 0.050 |
| Cut-off value of GPS | |||||
| 1 | 9 | 2.286 (1.533–3.409) | <0.001 | 61.9% | 0.007 |
| 2 | 7 | 3.506 (2.158–5.695) | <0.001 | 57.0% | 0.030 |
| Cancer-specific survival | |||||
| Sample size | |||||
| >500 | 1 | 1.280 (1.084–1.512) | |||
| ≤500 | 10 | 2.098 (1.612–2.729) | <0.001 | 48.9% | 0.0728 |
| Study of region | |||||
| Asia | 9 | 2.29 (1.697–3.090) | <0.001 | 46.8% | 0.082 |
| others | 2 | 1.315 (1.133–1.525) | <0.001 | 0% | <0.001 |
| Cut-off value of GPS | |||||
| 1 | 5 | 1.589 (1.216–2.076) | 0.001 | 56.5% | 0.057 |
| 2 | 6 | 2.365 (1.599–3.500) | <0.001 | 48.5% | 0.084 |
In addition, we explored the influence of GPS on survival in different disease stages. By grouping CRC patients into two subsets, 6 studies included patients with primary operable diseases (stages I,II, III), and 10 studies included patients with advanced inoperable diseases (stage IV). The rest included a mixed population and were excluded as the data was not able to be extracted according to the above two groups. The pooled estimate also showed that elevated GPS was correlated with worse survival, both in primary operable patients (HR = 2.43, 95% CI: 1.42–4.18, P = 0.001) (Fig. 2C) and advanced inoperable patients (HR = 2.08, 95% CI: 1.65–2.61, P < 0.001) (Fig. 2D).
GPS and clinicopathological features
There were 8 studies reporting the relationship between the level of GPS and TNM stage16–18, 20, 21, 23, 27, 29. The combined odds ratio (OR) of 1.44 (95% CI: 1.010–2.065, P = 0.044) displayed that patients with elevated GPS was prone to be in advanced TNM stages, with no significant heterogeneity (I 2 = 38.5%, P = 0.123; Fig. 3A). The relationship between GPS and serum carcino-embryonic antigen (CEA) level in CRC patients was presented in 10 studies16–19, 21, 24, 27, 29, 31, 33. The pooled OR was 2.252 (95% CI: 1.508–3.362, P = 0.000), indicating that elevated GPS was associated with elevated serum CEA, with no obvious heterogeneity (I 2 = 41.9%, P = 0.078; Fig. 3B). There was no significant correlation between increased GPS and lymphatic invasion (positive vs negative) and vascular invasion (positive vs negative) (Table 3).
Figure 3.
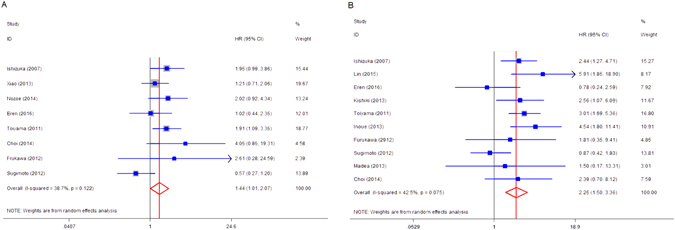
The forest plot between pretreatment GPS and clinicopathological parameters in CRC. (A) TNM stage (III, IV vs 0, I, II); (B) serum CEA.
Table 3.
Relationship between pretreatment GPS and lymphatic invasion and venous invasion.
| Factors | OR | 95% CI | P-value |
|---|---|---|---|
| Lymphatic invasion | 1.634 | 0.788–3.388 | 0.187 |
| Venous invasion | 1.295 | 0.846–1.981 | 0.233 |
Meta-regression analysis
In order to explore the source of heterogeneity, a meta-regression analysis was performed based on the following variables: sample size, study region, cut-off value of GPS and TNM stage. The result showed that for the 16 studies on OS, sample size was significantly related to heterogeneity (P = 0.001) (Fig. 4). Study region, cut-off value and TNM stage were negatively related to hazards of endpoints (Table 4). For 11 studies on CSS, study of region was significantly related to heterogeneity, while sample size, cut-off value and TNM stage were negatively related to hazards of endpoints (Table 4).
Figure 4.
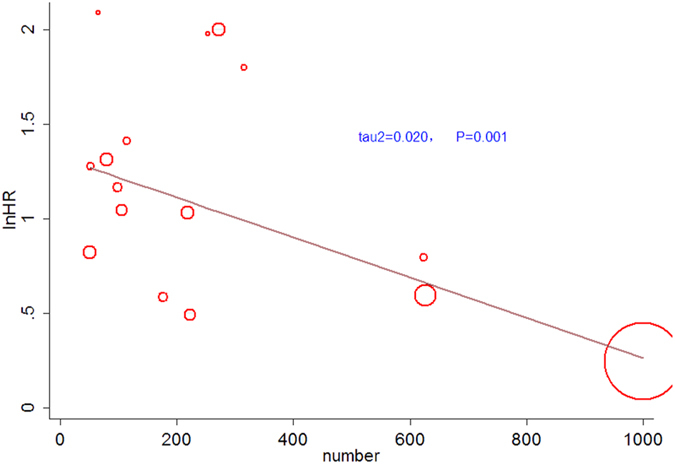
Meta-regression plot of HRs of OS against sample size.
Table 4.
Results of meta-regression on OS and CSS
| Variables | Coeffecient | Standard error | t | P value | 95% CI |
|---|---|---|---|---|---|
| Overall survival | |||||
| Cut-off value | 0.4296658 | 0.2943846 | 1.46 | 0.166 | −0.2017264, 1.061058 |
| Sample size | −0.001059 | 0.0002384 | −4.44 | 0.001 | −0.0015702, −0.0005477 |
| region | 0.2468601 | 0.3658075 | 0.67 | 0.515 | −0.5682097, 1.06193 |
| TNM stage | 0.7961549 | 0.7037753 | 1.13 | 0.291 | −0.8267539, 2.419064 |
| Cancer-specific survival | |||||
| Cut-off value | 0.3533804 | 0.2600391 | 1.36 | 0.207 | −0.234869, 0.9416297 |
| Sample size | −0.0005666 | 0.0004215 | −1.34 | 0.212 | −0.00152, 0.0003868 |
| region | 0.3859487 | 0.1528578 | 2.52 | 0.036 | 0.033458,0.7384393 |
| TNM stage | 0.6038852 | 0.4511961 | 1.34 | 0.223 | −0.4630241,1.670795 |
Sensitivity analysis
In order to assess whether the results were credible and stable with obvious heterogeneity, sensitivity analysis was carried out by means of omitting each study by turns (Fig. 5A and B). The result indicated that there was no obvious influence of one individual study on the pooled HR.
Figure 5.
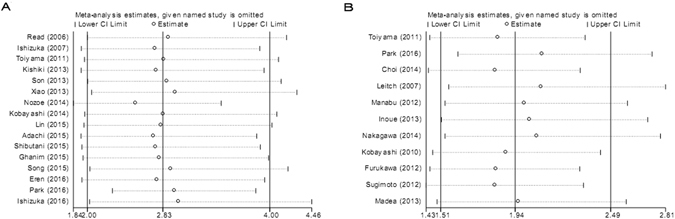
(A) Sensitivity analysis of 16 studies inclued in this meta-analysis for OS. (B) Sensitivity analysis of 11 studies inclued in this meta-analysis for CSS.
Publication bias
A funnel plot and Egger’s test were employed to investigate publication bias. As a result, for the studies of the GPS and OS, Begg’s test showed no publication bias (P = 0.260, Fig. 6A), whereas, Egger’s test revealed statistical significance (P < 0.001). For the studies of the GPS and CSS, substantial publication bias was detected both in the Begg’s test (P = 0.013, Fig. 6B) and Egger’s test (P < 0.001). Consequently, the “trim and fill” analysis was further performed and the recalculated result did not change significantly (OS: HR = 1.381, 95% CI: 1.247–1.529, P < 0.001; CSS: HR = 1.430, 95% CI: 1.279–1.599, P < 0.001), indicating the stability of our pooled results.
Figure 6.
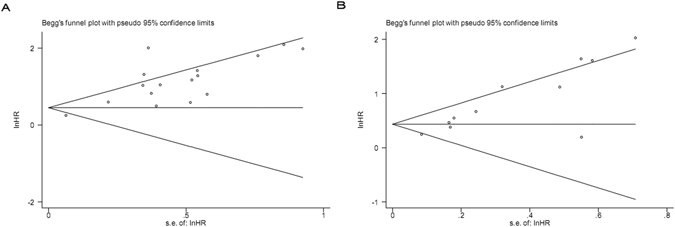
(A) Funnel plot of 16 included studies in this meta-analysis for OS; (B) Funnel plot of 11 included studies in this meta-analysis for CSS.
Discussion
Inflammation plays an important role in the development and progression of various tumours. Cancer can induce local or systematic inflammation, mediated by the activation of transcription factors and release of cytokines, which can inversely influence tumour activities, including cell proliferation, angiogenesis, cell migration, and invasion39, 40. There are several inflammation-based prognostic systems having been reported in cancers. GPS is one of them, combining serum CRP and albumin which are closely connected with the prognosis of cancer patients respectively41, 42. Initially, GPS was applied to determine the prognosis of patients with inoperative lung cancer8. Thereafter, it was increasingly used to assess the outcome of patients with multiple malignant tumours43–47. Recently, a number of investigations attempted to evaluate the significance of GPS in predicting patients’ survival in CRC14–38, but had the inconsistent results.
The current study, to our best knowledge, is the most comprehensive meta-analysis assessing the correlation between GPS and the prognosis of patients with CRC. A total of 25 studies containing 5660 CRC patients were included. As shown in this meta-analysis, the pooled HR of OS and CSS were 2.83 (95% CI: 2.00–4.00, P < 0.001) and 1.94 (95% CI: 1.51–2.49, P < 0.001) respectively, which suggested that patients with elevated GPS were predisposed to exhibit poor survival outcome. When subgroup analysis was performed stratifying by sample size, study of region and cut-off value of GPS, worse survival was also presented in CRC patients with elevated GPS in all the subgroups, which suggested that our results were reliable.
As staging is currently the most important prognostic indicator for CRC, we grouped patients into two subsets–the primary operable diseases and the advanced inoperable diseases. Then we conducted meta-analysis in these two groups respectively, regarding the information with reference to GPS and survival outcome. Inspiringly, the pooled results were consistent in the two groups, despite their different presentations, diagnosis and treatments, both suggesting that elevated GPS was associated with worse survival in CRC patients, which further validated our conclusion.
Furthermore, an obvious relationship between GPS and clinical parameters, including TNM stage and serum CEA level was observed, which further confirmed that GPS could be a promising predicting index for CRC patients. However, we found no significant correlation between the increased GPS and lymphatic and venous invasion. One possible explanation could be that among the included studies, only 8 studies had reported the connection between GPS and lymphatic invasion, 9 between GPS and venous invasion, resulting in a small sample size for analyzing.
The forest plot revealed heterogeneity in this meta-analysis (I 2 = 74.3%, P < 0.001; I 2 = 63.3%, P = 0.002). Therefore, we performed meta-regression analysis to explore the source. The results indicated that the sample size might contribute to the heterogeneity across 16 studies on OS, while the region of study contributed to heterogeneity across 11 studies on CSS. Among the included 25 studies, only 3 contained with populations more than five hundreds. A small sample size was usually considered as the source of heterogeneity. Of note, the majority of the included studies (19/25) were conducted in Asian medical institutions, and ethnic background and life styles may contribute to the variations in cancer patients’ prognosis, which was consistent with other meta-analysis48. However, the subgroup analysis in terms of the sample size and study region did not alter the overall results.
There are several limitations that should be considered in this meta-analysis. First of all, though the amount of included studies was large, significant interstudy heterogeneity was still observed and could not be eliminated completely. The variables included in our meta-regression analysis partly explained the heterogeneity, other factors might affect the prognosis. Secondly, most of the included studies were retrospective. Thus, further larger scale, well-designed prospective investigations are required in the future.
In conclusion, current evidence from the meta-analysis identifies elevated GPS as a promising prognostic biomarker in CRC. GPS, calculated from two conventional laboratory data, does not demand additional examinations such as imaging techniques or histopathology, and could be easily obtained from peripheral blood test. Additionally, compared to the existing evaluation system such as TNM stage, GPS could be obtained before operation. Pre-operative elevated GPS suggests patients at high risk of recurrence and poor survival, which could help doctors to adopt more careful surgery and more rigorous follow-up.
Materials and Methods
Literature search
We performed electronic literature searches using Pubmed, Embase, Cochrane library, Web of Science, ChinaInfo and Chinese National Knowledge Infrastructure before June 01, 2016. Following key search terms were included in our study: “Glasgow Prognostic Score”, “GPS”, “prognosis”, “colorectal carcinoma”, “colon cancer”. The references of all included studies were screened to identify additional related full articles.
Study selection
A study was considered eligible according to the following criteria: (1) the study was written in English or Chinese; (2) the diagnosis of CRC was confirmed by pathology; (3) correlation between GPS and overall survival (OS)/cancer-specific (CSS) was presented in the article; (4) HRs with its 95% CI were reported in the study. Exclusion criteria was defined as following: non-human CRC studies; studies published in abstracts, letters, reviews, case reports, expert comments, duplicated articles, and studies without HR and 95% CI.
Data extraction
Two investigators (LYY and HXK) independently extracted data from the eligible studies and disagreements were resolved by discussion. The following items were obtained from each study: (1) name of the first author, study region, publication year, number of cases, age, dominating treatment, follow-up period; (2) clinicopathological parameters including TNM stage, lymphatic invasion, venous invasion, lymph node metastasis, and carcinoembryonic antigen (CEA) level; (3) survival data of OS and CSS; (4) the cut-off value of GPS.
Quality assessment
We evaluated the articles identified by the above criteria and implemented quality assessment according to the Newcastle-Ottawa-Scale (NOS)49. Any discrepancy was resolved by consensus.
Statistical analysis
Data analysis was conducted using Stata 12.0 (Stata Corporation, Texas, US). The HRs with its 95% CI was directly obtained from each literature and the pooled HR was used to assess the significance of GPS on OS and CSS of the patients with CRC. If several estimates were reported for the same value, HRs and 95% CI were extracted preferentially from multivariate analysis where available. Otherwise, HRs were extracted from univariate analysis. For analyzing the correlation between GPS and clinicopathological parameters, OR with its 95% CI was combined by Mantel-Haenszel method as the effective value. Heterogeneity among pooled results was assessed using Cochrane Q test and Higgin I 2 statistic. P < 0.05 or/and I 2 > 50% were considered statistically heterogeneous and the random-effect model was applied; otherwise, the fixed-effect model was performed. Publication bias was evaluated using the funnel plot and Egger’s test (values of P > 0.05 indicated lack of publication bias)50. Meta-regression analysis was conducted to explore potential sources of heterogeneity. We also performed a sensitivity analysis in which one study at a time was removed and the rest were analyzed to evaluate whether the results could have been affected markedly by a single study.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (81472214), the Zhejiang province key science and technology innovation team (2013TD13).
Author Contributions
All authors contributed significantly to this work. Y.Y.L., X.K.H., and L.J.W. designed the research study. Y.Y.L. performed the research study and collected the data; X.K.H. analyzed the data; Y.Y.L. wrote the first draft of the manuscript; J.P. and S.J.C. revised the manuscript. All authors reviewed and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Burden of Disease Cancer Collaboration et al. The global burden of cancer 2013. JAMA Oncol. 2013;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Sobin, L. H., Gospodarowicz, M. k. & Wittekind, C. TNM classification of malignant tumors, 7th ed. New York: Wiley-Blackwell, 73–77 (2011).
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110:1409–1412. doi: 10.1038/bjc.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laird BJ, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res. 2013;19:5456–5464. doi: 10.1158/1078-0432.CCR-13-1066. [DOI] [PubMed] [Google Scholar]
- 8.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic infammatory response in patients with inoperable non-small-cell lung cancer. Brit J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizuka M, et al. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am J Surg. 2012;203:101–106. doi: 10.1016/j.amjsurg.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Vashist YK, et al. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18:1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang CX, et al. Association between pretreatment glasgow prognostic score and gastric cancer survival and clinicopathological features: a meta-analysis. OncoTargets and therapy. 2016;9:3883. doi: 10.2147/OTT.S103996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score inpatients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106:279–83. doi: 10.1038/bjc.2011.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torre ML, et al. The Glasgow Prognostic Score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 14.Leitch EF, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97:1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of patients with nonmetastatic colon cancer. Annals of surgical oncology. 2013;20:2908–2913. doi: 10.1245/s10434-013-2968-8. [DOI] [PubMed] [Google Scholar]
- 16.Read JA, Choy STB, Beale PJ, Clarke SJ. Evaluation of Nutritional and Inflammatory Status of Advanced Colorectal Cancer Patients and Its Correlation With Survival. Nutrition and Cancer. 2006;55:78–85. doi: 10.1207/s15327914nc5501_10. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Annals of surgery. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, et al. Elevated C-reactive protein and hypoalbuminemia measured before resection of colorectal liver metastases predict postoperative survival. Digestive surgery. 2010;27:285–290. doi: 10.1159/000280021. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa K, et al. The Glasgow prognostic score is valuable for colorectal cancer with both synchronous and metachronous unresectable liver metastases. Oncology letters. 2012;4:324–328. doi: 10.3892/ol.2012.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimoto K, et al. Glasgow prognostic score as a prognostic factor in patients undergoing curative surgery for colorectal cancer. Digestive surgery. 2012;29:503–509. doi: 10.1159/000346002. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K, et al. Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer research. 2013;33:5567–5573. [PubMed] [Google Scholar]
- 22.Xiao CH, et al. Relationship between Glasgow Prognostic Score, liver metastasis and prognosis of rectal cancer. Chin J Bases Clin General Surg. 2013;20:620–626. [Google Scholar]
- 23.Choi KW, et al. Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Annals of surgical treatment and research. 2014;86:309–313. doi: 10.4174/astr.2014.86.6.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, et al. Combined evaluation of the Glasgow prognostic score and carcinoembryonic antigen concentration prior to hepatectomy predicts postoperative outcomes in patients with liver metastasis from colorectal cancer. Hepato-gastroenterology. 2014;61:1359–1362. [PubMed] [Google Scholar]
- 25.Nozoe T, Matono R, Ijichi H, Ohga T, Ezaki T. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. International surgery. 2014;99:512–517. doi: 10.9738/INTSURG-D-13-00118.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin MS, Huang JX, Yu H. Prognostic significance of Glasgow prognostic score in patients with stage II colorectal cancer. International journal of clinical and experimental medicine. 2015;8:19138–19143. [PMC free article] [PubMed] [Google Scholar]
- 27.Shibutani M, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World journal of surgical oncology. 2015;13:1–8. doi: 10.1186/s12957-015-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizuka M, et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Annals of surgical oncology. 2016;23:900–907. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 29.Eren T, et al. Clinical Significance of the Glasgow Prognostic Score for Survival after Colorectal Cancer Surgery. Journal of Gastrointestinal Surgery. 2016;20:1231–1238. doi: 10.1007/s11605-016-3114-2. [DOI] [PubMed] [Google Scholar]
- 30.Toiyama YJ, et al. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Experimental and therapeutic medicine. 2011;2:95–101. doi: 10.3892/etm.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto M, et al. Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology. 2012;83:10–15. doi: 10.1159/000338329. [DOI] [PubMed] [Google Scholar]
- 32.Kishiki T, et al. Modified Glasgow prognostic score in patients with incurable stage IV colorectal cancer. The American Journal of Surgery. 2013;206:234–240. doi: 10.1016/j.amjsurg.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Inoue Y, et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology. 2013;84:100–107. doi: 10.1159/000343822. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa K, et al. The modified Glasgow prognostic score as a predictor of survival after hepatectomy for colorectal liver metastases. Annals of surgical oncology. 2014;21:1711–1718. doi: 10.1245/s10434-013-3342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adachi T, et al. The modified Glasgow prognostic score for early mortality in patients with synchronous peritoneal carcinomatosis from colorectal cancer. Surgery today. 2015;45:1396–1403. doi: 10.1007/s00595-014-1080-4. [DOI] [PubMed] [Google Scholar]
- 36.Ghanim B, et al. Elevated inflammatory parameters and inflammation scores are associated with poor prognosis in patients undergoing pulmonary metastasectomy for colorectal cancer. Interactive Cardio Vascular and Thoracic Surgery. 2015;21:616–623. doi: 10.1093/icvts/ivv206. [DOI] [PubMed] [Google Scholar]
- 37.Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World journal of gastroenterology. 2015;21:12410–12420. doi: 10.3748/wjg.v21.i43.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Annals of surgery. 2016;263:326–336. doi: 10.1097/SLA.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 41.McMillan DC. Systemic inlammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 42.Jing X, et al. Association between serum C-reactive protein value and prognosis of patients with non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:10633–10639. [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang X, et al. Prognostic importance of the inflammation-based Glasgow Prognostic Score in patients with gastric cancer. Br J Cancer. 2012;107:275–279. doi: 10.1038/bjc.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Wang H, Liu CC, Lu Y, Tang H. The Glasgow Prognostic Score (GPS) is a novel prognostic indicator in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer Res Clin Oncol. 2016;142:2339–2345. doi: 10.1007/s00432-016-2228-y. [DOI] [PubMed] [Google Scholar]
- 45.Yuksel OH, et al. Preoperative Glasgow prognostic score as a predictor of primary bladder cancer recurrence. Mol Clin Oncol. 2016;5:201–206. doi: 10.3892/mco.2016.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yotsukura M, et al. Value of the Glasgow Prognostic Score as a Prognostic Factor in Resectable Non-Small Cell Lung Cancer. J Thorac Oncol. 2016;11:1311–1318. doi: 10.1016/j.jtho.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Eren T, et al. Clinical significance of the Glasgow Prognostic Score for survival after colorectal cancer surgery. J Gastrointest Surg. 2016;20:1231–1238. doi: 10.1007/s11605-016-3114-2. [DOI] [PubMed] [Google Scholar]
- 48.Li MX, et al. Prognostic Role of Glasgow Prognostic Score in Patients With Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Medicine. 2015;94:e2133. doi: 10.1097/MD.0000000000002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 50.Egger, M., Davey, Smith, G., Schneide, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed]


